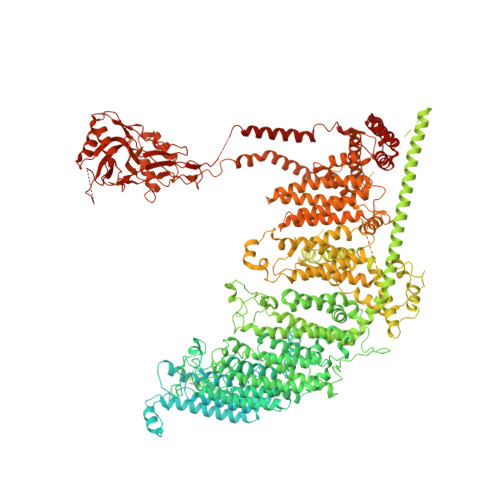
Structure-based membrane dome mechanism for Piezo mechanosensitivity.
Guo, Y.R., MacKinnon, R.(2017) Elife 6
- PubMed: 29231809
- DOI: https://doi.org/10.7554/eLife.33660
- Primary Citation of Related Structures:
6B3R - PubMed Abstract:
Mechanosensitive ion channels convert external mechanical stimuli into electrochemical signals for critical processes including touch sensation, balance, and cardiovascular regulation. The best understood mechanosensitive channel, MscL, opens a wide pore, which accounts for mechanosensitive gating due to in-plane area expansion. Eukaryotic Piezo channels have a narrow pore and therefore must capture mechanical forces to control gating in another way. We present a cryo-EM structure of mouse Piezo1 in a closed conformation at 3.7Å-resolution. The channel is a triskelion with arms consisting of repeated arrays of 4-TM structural units surrounding a pore. Its shape deforms the membrane locally into a dome. We present a hypothesis in which the membrane deformation changes upon channel opening. Quantitatively, membrane tension will alter gating energetics in proportion to the change in projected area under the dome. This mechanism can account for highly sensitive mechanical gating in the setting of a narrow, cation-selective pore.
- Laboratory of Molecular Neurobiology and Biophysics, Howard Hughes Medical Institute, The Rockefeller University, New York, United States.
Organizational Affiliation: