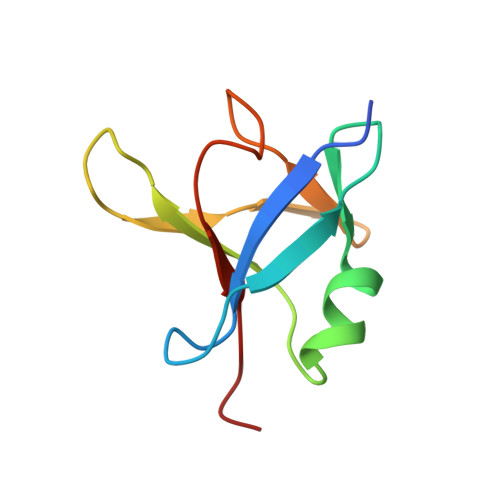
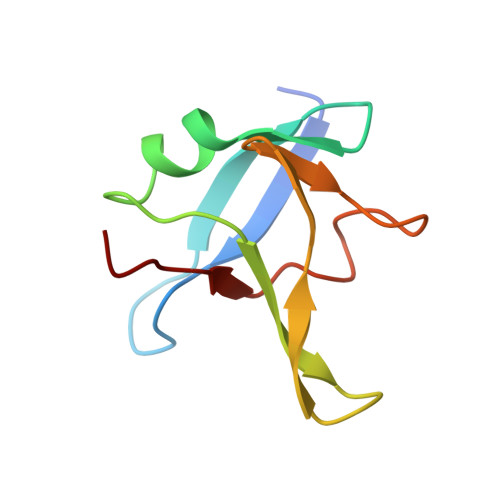
A single N-terminal phosphomimic disrupts TDP-43 polymerization, phase separation, and RNA splicing.
Wang, A., Conicella, A.E., Schmidt, H.B., Martin, E.W., Rhoads, S.N., Reeb, A.N., Nourse, A., Ramirez Montero, D., Ryan, V.H., Rohatgi, R., Shewmaker, F., Naik, M.T., Mittag, T., Ayala, Y.M., Fawzi, N.L.(2018) EMBO J 37
- PubMed: 29438978
- DOI: https://doi.org/10.15252/embj.201797452
- Primary Citation of Related Structures:
6B1G - PubMed Abstract:
TDP-43 is an RNA-binding protein active in splicing that concentrates into membraneless ribonucleoprotein granules and forms aggregates in amyotrophic lateral sclerosis (ALS) and Alzheimer's disease. Although best known for its predominantly disordered C-terminal domain which mediates ALS inclusions, TDP-43 has a globular N-terminal domain (NTD). Here, we show that TDP-43 NTD assembles into head-to-tail linear chains and that phosphomimetic substitution at S48 disrupts TDP-43 polymeric assembly, discourages liquid-liquid phase separation (LLPS) in vitro , fluidizes liquid-liquid phase separated nuclear TDP-43 reporter constructs in cells, and disrupts RNA splicing activity. Finally, we present the solution NMR structure of a head-to-tail NTD dimer comprised of two engineered variants that allow saturation of the native polymerization interface while disrupting higher-order polymerization. These data provide structural detail for the established mechanistic role of the well-folded TDP-43 NTD in splicing and link this function to LLPS. In addition, the fusion-tag solubilized, recombinant form of TDP-43 full-length protein developed here will enable future phase separation and in vitro biochemical assays on TDP-43 function and interactions that have been hampered in the past by TDP-43 aggregation.
- Department of Molecular Pharmacology, Physiology, and Biotechnology, Brown University, Providence, RI, USA.
Organizational Affiliation: