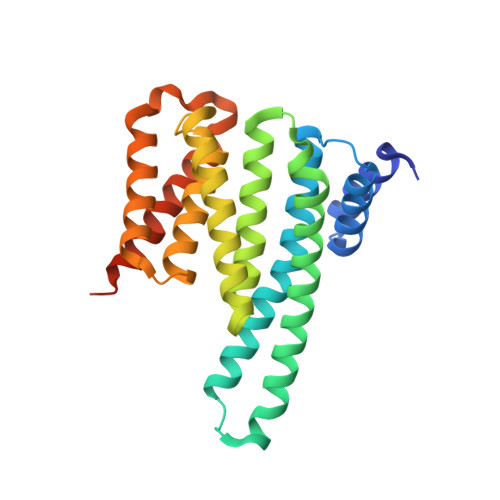
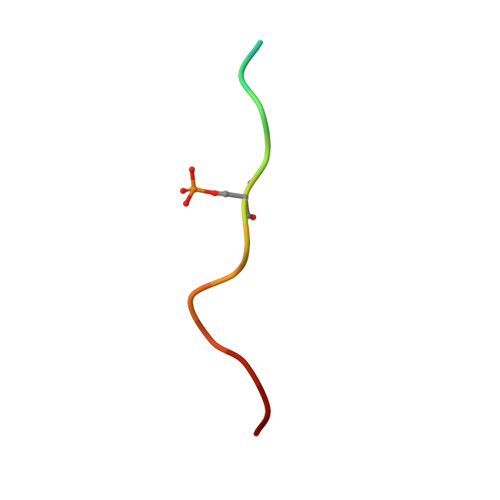
YWHA/14-3-3 proteins recognize phosphorylated TFEB by a noncanonical mode for controlling TFEB cytoplasmic localization.
Xu, Y., Ren, J., He, X., Chen, H., Wei, T., Feng, W.(2019) Autophagy 15: 1017-1030
- PubMed: 30653408
- DOI: https://doi.org/10.1080/15548627.2019.1569928
- Primary Citation of Related Structures:
6A5Q, 6A5S - PubMed Abstract:
As a master regulator of the macroautophagy/autophagy-lysosomal pathway, TFEB (transcription factor EB) plays a prominent role in regulating neurodegenerative diseases and cancer. The transcription activity of TFEB is tightly controlled by phosphorylation and dephosphorylation. Phosphorylated S211 (p-S211) of TFEB can be recognized by YWHA/14-3-3 proteins for TFEB cytoplasmic localization. Here, we characterized the interactions between phosphorylated TFEB and YWHA/14-3-3 proteins and determined the structures of YWHA/14-3-3 proteins in complex with a TFEB p-S211-peptide. Although the critical arginine for YWHA/14-3-3 recognition is missing in the N terminus of the TFEB p-S211-peptide, the C-terminal additional hydrophobic residues of the peptide unexpectedly occupy nearly half of the target-binding groove of YWHA/14-3-3 proteins, which compensates for the N-terminal defect and is distinct from the canonical YWHA/14-3-3-binding mode. Mutations of essential residues in the interaction interface between TFEB and YWHA/14-3-3 proteins disrupted their interactions and severely impaired the cytoplasmic localization of TFEB, which altered the expression of TFEB target genes and affected autophagy. Thus, YWHA/14-3-3 proteins recognize phosphorylated TFEB by a noncanonical mode for controlling TFEB cytoplasmic localization and its activity. Abbreviation: ACTB: actin beta; ALP: autophagy-lysosomal pathway; ATP6V1H: ATPase H + transporting V1 subunit H; bHLH: basic helix-loop-helix; CLEAR: coordinated lysosomal expression and regulation; Co-IP: co-immunoprecipitation; CTSB: cathepsin B; CTSD: cathepsin D; LAMP1: lysosomal associated membrane protein 1; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MITF: melanocyte inducing transcription factor; NLS: nuclear localization signal; TFEB: transcription factor EB; YWHA/14-3-3: tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein.
- a National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics , Chinese Academy of Sciences , Beijing , China.
Organizational Affiliation: