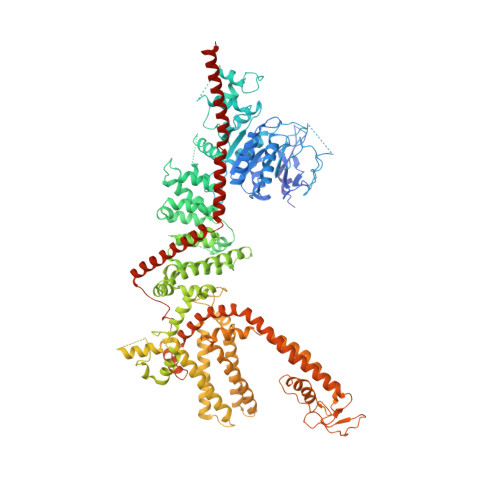
Electron cryo-microscopy structure of a human TRPM4 channel.
Winkler, P.A., Huang, Y., Sun, W., Du, J., Lu, W.(2017) Nature 552: 200-204
- PubMed: 29211723
- DOI: https://doi.org/10.1038/nature24674
- Primary Citation of Related Structures:
5WP6 - PubMed Abstract:
Ca 2+ -activated, non-selective (CAN) ion channels sense increases of the intracellular Ca 2+ concentration, producing a flux of Na + and/or K + ions that depolarizes the cell, thus modulating cellular Ca 2+ entry. CAN channels are involved in cellular responses such as neuronal bursting activity and cardiac rhythm. Here we report the electron cryo-microscopy structure of the most widespread CAN channel, human TRPM4, bound to the agonist Ca 2+ and the modulator decavanadate. Four cytosolic C-terminal domains form an umbrella-like structure with a coiled-coil domain for the 'pole' and four helical 'ribs' spanning the N-terminal TRPM homology regions (MHRs), thus holding four subunits in a crown-like architecture. We observed two decavanadate-binding sites, one in the C-terminal domain and another in the intersubunit MHR interface. A glutamine in the selectivity filter may be an important determinant of monovalent selectivity. Our structure provides new insights into the function and pharmacology of both the CAN and the TRPM families.
- Van Andel Institute, 333 Bostwick Avenue N.E., Grand Rapids, Michigan 49503, USA.
Organizational Affiliation: