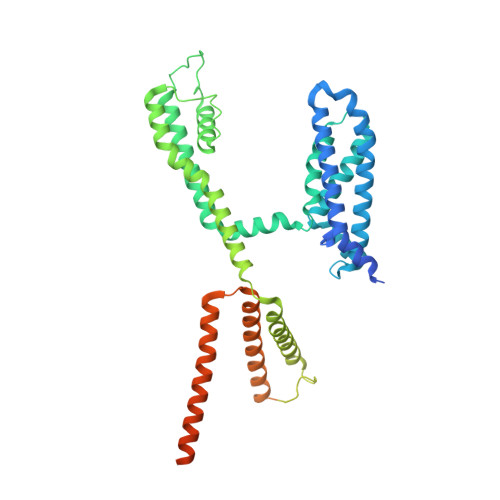
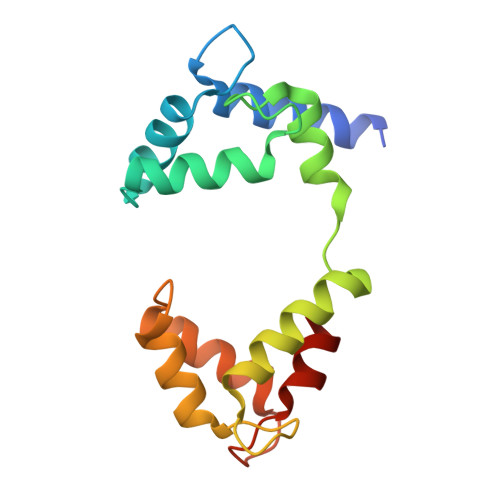
Cryo-EM Structure of a KCNQ1/CaM Complex Reveals Insights into Congenital Long QT Syndrome.
Sun, J., MacKinnon, R.(2017) Cell 169: 1042-1050.e9
- PubMed: 28575668
- DOI: https://doi.org/10.1016/j.cell.2017.05.019
- Primary Citation of Related Structures:
5VMS - PubMed Abstract:
KCNQ1 is the pore-forming subunit of cardiac slow-delayed rectifier potassium (I Ks ) channels. Mutations in the kcnq1 gene are the leading cause of congenital long QT syndrome (LQTS). Here, we present the cryoelectron microscopy (cryo-EM) structure of a KCNQ1/calmodulin (CaM) complex. The conformation corresponds to an "uncoupled," PIP 2 -free state of KCNQ1, with activated voltage sensors and a closed pore. Unique structural features within the S4-S5 linker permit uncoupling of the voltage sensor from the pore in the absence of PIP 2 . CaM contacts the KCNQ1 voltage sensor through a specific interface involving a residue on CaM that is mutated in a form of inherited LQTS. Using an electrophysiological assay, we find that this mutation on CaM shifts the KCNQ1 voltage-activation curve. This study describes one physiological form of KCNQ1, depolarized voltage sensors with a closed pore in the absence of PIP 2 , and reveals a regulatory interaction between CaM and KCNQ1 that may explain CaM-mediated LQTS.
- Laboratory of Molecular Neurobiology and Biophysics and Howard Hughes Medical Institute, The Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.
Organizational Affiliation: