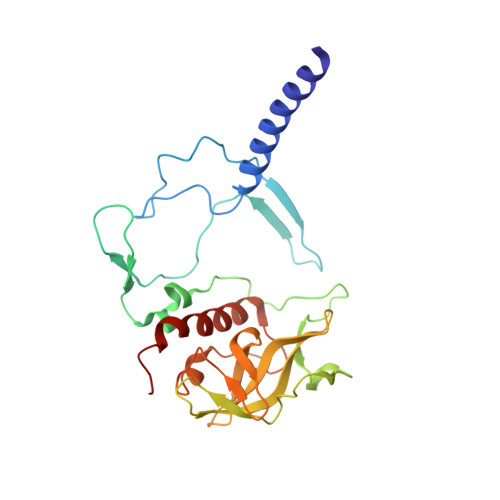
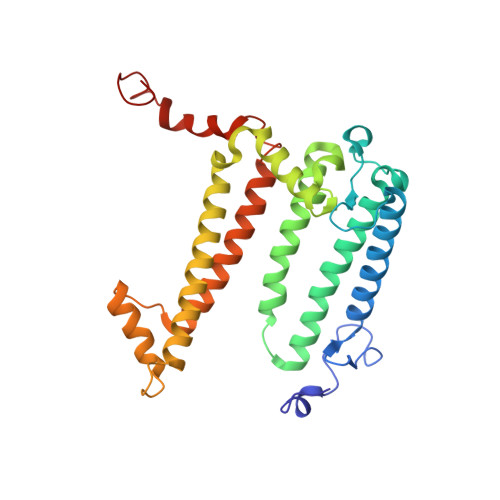
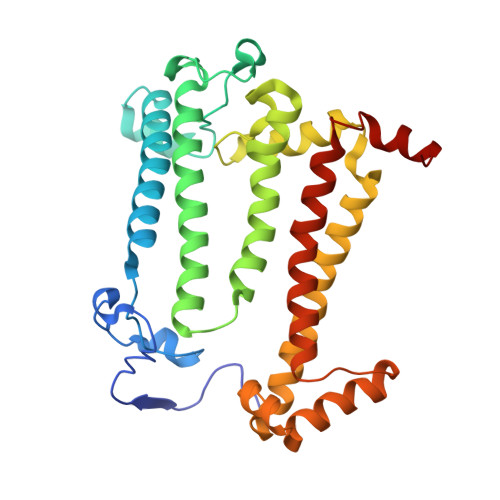
X-ray transparent microfluidic chips for high-throughput screening and optimization of in meso membrane protein crystallization.
Schieferstein, J.M., Pawate, A.S., Sun, C., Wan, F., Sheraden, P.N., Broecker, J., Ernst, O.P., Gennis, R.B., Kenis, P.J.A.(2017) Biomicrofluidics 11: 024118-024118
- PubMed: 28469762
- DOI: https://doi.org/10.1063/1.4981818
- Primary Citation of Related Structures:
5V33 - PubMed Abstract:
Elucidating and clarifying the function of membrane proteins ultimately requires atomic resolution structures as determined most commonly by X-ray crystallography. Many high impact membrane protein structures have resulted from advanced techniques such as in meso crystallization that present technical difficulties for the set-up and scale-out of high-throughput crystallization experiments. In prior work, we designed a novel, low-throughput X-ray transparent microfluidic device that automated the mixing of protein and lipid by diffusion for in meso crystallization trials. Here, we report X-ray transparent microfluidic devices for high-throughput crystallization screening and optimization that overcome the limitations of scale and demonstrate their application to the crystallization of several membrane proteins. Two complementary chips are presented: (1) a high-throughput screening chip to test 192 crystallization conditions in parallel using as little as 8 nl of membrane protein per well and (2) a crystallization optimization chip to rapidly optimize preliminary crystallization hits through fine-gradient re-screening. We screened three membrane proteins for new in meso crystallization conditions, identifying several preliminary hits that we tested for X-ray diffraction quality. Further, we identified and optimized the crystallization condition for a photosynthetic reaction center mutant and solved its structure to a resolution of 3.5 Å.
- Department of Chemical and Biomolecular Engineering, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA.
Organizational Affiliation: