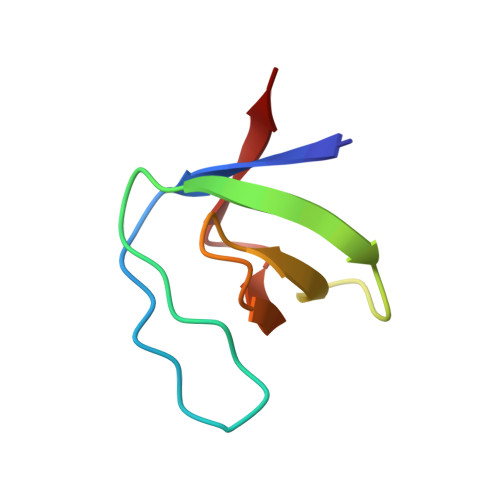
The Molecular Mechanisms Underlying the Hijack of Host Proteins by the 1918 Spanish Influenza Virus.
Shen, Q., Zeng, D., Zhao, B., Bhatt, V.S., Li, P., Cho, J.H.(2017) ACS Chem Biol 12: 1199-1203
- PubMed: 28368102
- DOI: https://doi.org/10.1021/acschembio.7b00168
- Primary Citation of Related Structures:
5UL6 - PubMed Abstract:
The 1918 Spanish influenza A virus (IAV) caused one of the most serious pandemics in history. The nonstructural protein 1 (NS1) of the 1918 IAV hijacks the interaction between human CrkII and JNK1. Little is, however, known about its molecular mechanism. Here, we performed X-ray crystallography, NMR relaxation dispersion experiment, and fluorescence spectroscopy to determine the structural, kinetic, and thermodynamic mechanisms underlying the hijacking of CrkII by 1918 IAV NS1. We observed that the interaction between a proline-rich motif in NS1 and the N-terminal SH3 domain of CrkII displays strikingly rapid kinetics and exceptionally high affinity with 100-fold faster k on and 3300-fold lower K d compared to those for the CrkII-JNK1 interaction. These results provide molecular insight into the mechanism by which 1918 IAV NS1 hijacks CrkII and disrupts its interactions with critical cellular signaling proteins.
- Department of Biochemistry and Biophysics, Texas A&M University , College Station, Texas 77843, United States.
Organizational Affiliation: