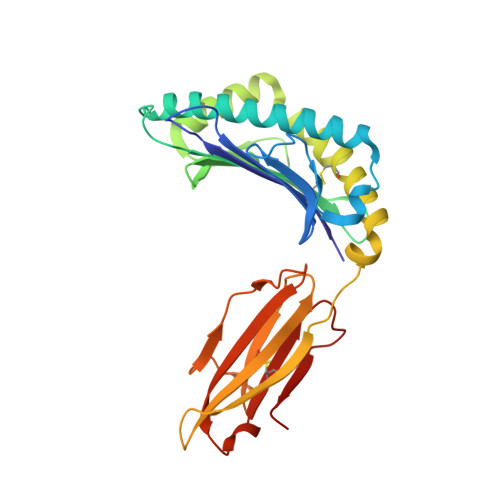
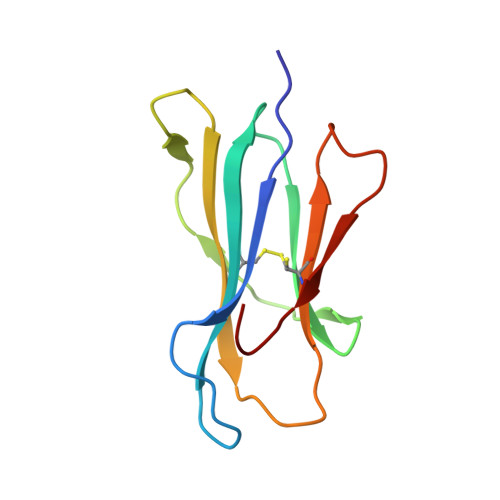
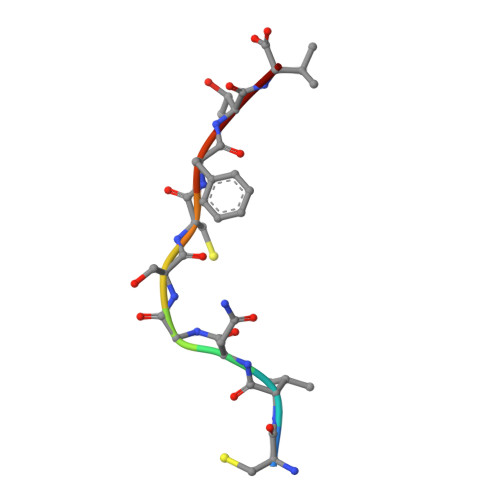
Lack of Heterologous Cross-reactivity toward HLA-A*02:01 Restricted Viral Epitopes Is Underpinned by Distinct alpha beta T Cell Receptor Signatures.
Grant, E.J., Josephs, T.M., Valkenburg, S.A., Wooldridge, L., Hellard, M., Rossjohn, J., Bharadwaj, M., Kedzierska, K., Gras, S.(2016) J Biological Chem 291: 24335-24351
- PubMed: 27645996
- DOI: https://doi.org/10.1074/jbc.M116.753988
- Primary Citation of Related Structures:
5SWQ - PubMed Abstract:
αβT cell receptor (TCR) genetic diversity is outnumbered by the quantity of pathogenic epitopes to be recognized. To provide efficient protective anti-viral immunity, a single TCR ideally needs to cross-react with a multitude of pathogenic epitopes. However, the frequency, extent, and mechanisms of TCR cross-reactivity remain unclear, with conflicting results on anti-viral T cell cross-reactivity observed in humans. Namely, both the presence and lack of T cell cross-reactivity have been reported with HLA-A*02:01-restricted epitopes from the Epstein-Barr and influenza viruses (BMLF-1 and M1 58 , respectively) or with the hepatitis C and influenza viruses (NS3 1073 and NA 231 , respectively). Given the high sequence similarity of these paired viral epitopes (56 and 88%, respectively), the ubiquitous nature of the three viruses, and the high frequency of the HLA-A*02:01 allele, we selected these epitopes to establish the extent of T cell cross-reactivity. We combined ex vivo and in vitro functional assays, single-cell αβTCR repertoire sequencing, and structural analysis of these four epitopes in complex with HLA-A*02:01 to determine whether they could lead to heterologous T cell cross-reactivity. Our data show that sequence similarity does not translate to structural mimicry of the paired epitopes in complexes with HLA-A*02:01, resulting in induction of distinct αβTCR repertoires. The differences in epitope architecture might be an obstacle for TCR recognition, explaining the lack of T cell cross-reactivity observed. In conclusion, sequence similarity does not necessarily result in structural mimicry, and despite the need for cross-reactivity, antigen-specific TCR repertoires can remain highly specific.
- From the Department of Microbiology and Immunology, University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Parkville, Victoria 3010, Australia.
Organizational Affiliation: