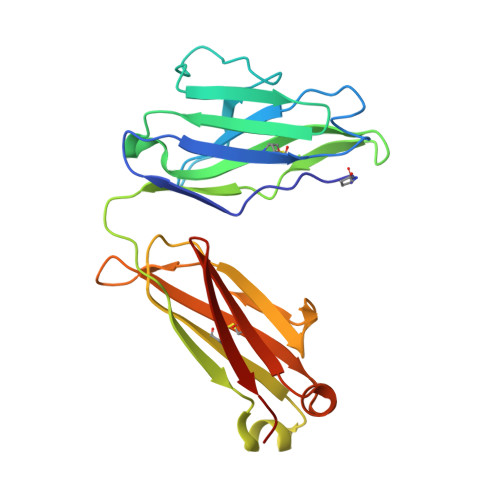
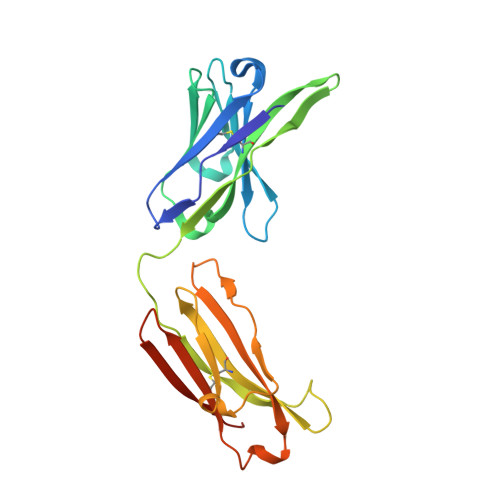
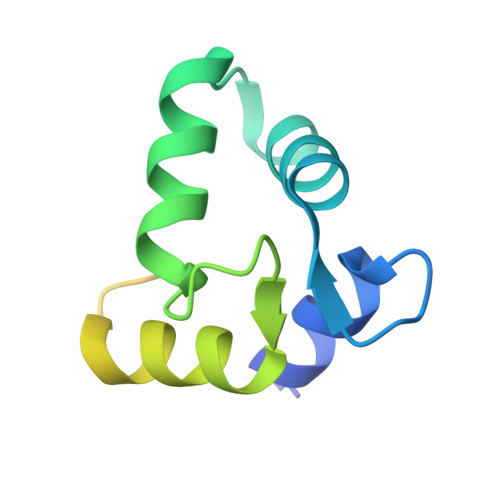
Structure of a patient-derived antibody in complex with allergen reveals simultaneous conventional and superantigen-like recognition.
Mitropoulou, A.N., Bowen, H., Dodev, T.S., Davies, A.M., Bax, H.J., Beavil, R.L., Beavil, A.J., Gould, H.J., James, L.K., Sutton, B.J.(2018) Proc Natl Acad Sci U S A 115: E8707-E8716
- PubMed: 30150373
- DOI: https://doi.org/10.1073/pnas.1806840115
- Primary Citation of Related Structures:
5OTJ - PubMed Abstract:
Antibodies classically bind antigens via their complementarity-determining regions, but an alternative mode of interaction involving V-domain framework regions has been observed for some B cell "superantigens." We report the crystal structure of an antibody employing both modes of interaction simultaneously and binding two antigen molecules. This human antibody from an allergic individual binds to the grass pollen allergen Phl p 7. Not only are two allergen molecules bound to each antibody fragment (Fab) but also each allergen molecule is bound by two Fabs: One epitope is recognized classically, the other in a superantigen-like manner. A single allergen molecule thus cross-links two identical Fabs, contrary to the one-antibody-one-epitope dogma, which dictates that a dimeric allergen at least is required for this to occur. Allergens trigger immediate hypersensitivity reactions by cross-linking receptor-bound IgE molecules on effector cells. We found that monomeric Phl p 7 induced degranulation of basophils sensitized solely with this monoclonal antibody expressed as an IgE, demonstrating that the dual specificity has functional consequences. The monomeric state of Phl p 7 and two structurally related allergens was confirmed by size-exclusion chromatography and multiangle laser light scattering, and the results were supported by degranulation studies with the related allergens, a second patient-derived allergen-specific antibody lacking the nonclassical binding site, and mutagenesis of the nonclassically recognized allergen epitope. The antibody dual reactivity and cross-linking mechanism not only have implications for understanding allergenicity and allergen potency but, importantly, also have broader relevance to antigen recognition by membrane Ig and cross-linking of the B cell receptor.
- Randall Centre for Cell and Molecular Biophysics, King's College London, SE1 1UL London, United Kingdom.
Organizational Affiliation: