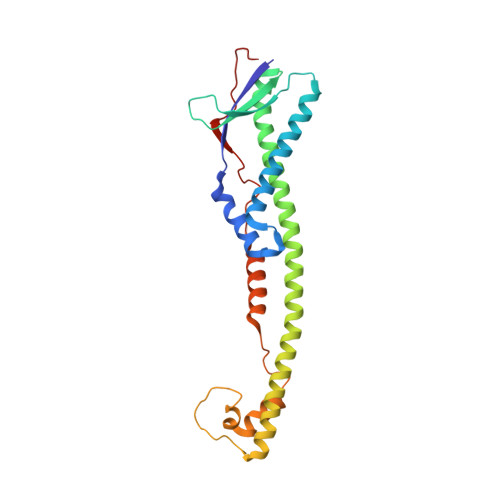
Full-length, Oligomeric Structure of Wzz Determined by Cryoelectron Microscopy Reveals Insights into Membrane-Bound States.
Collins, R.F., Kargas, V., Clarke, B.R., Siebert, C.A., Clare, D.K., Bond, P.J., Whitfield, C., Ford, R.C.(2017) Structure 25: 806-815.e3
- PubMed: 28434914
- DOI: https://doi.org/10.1016/j.str.2017.03.017
- Primary Citation of Related Structures:
5NBZ - PubMed Abstract:
Wzz is an integral inner membrane protein involved in regulating the length of lipopolysaccharide O-antigen glycans and essential for the virulence of many Gram-negative pathogens. In all Wzz homologs, the large periplasmic domain is proposed to be anchored by two transmembrane helices, but no information is available for the transmembrane and cytosolic domains. Here we have studied purified oligomeric Wzz complexes using cryoelectron microscopy and resolved the transmembrane regions within a semi-continuous detergent micelle. The transmembrane helices of each monomer display a right-handed super-helical twist, and do not interact with the neighboring transmembrane domains. Modeling, flexible fitting and multiscale simulation approaches were used to study the full-length complex and to provide explanations for the influence of the lipid bilayer on its oligomeric status. Based on structural and in silico observations, we propose a new mechanism for O-antigen chain-length regulation that invokes synergy of Wzz and its polymerase partner, Wzy.
- Faculty of Biology, Medicine and Health, The University of Manchester, Dover Street, Manchester M13 9PT, UK.
Organizational Affiliation: