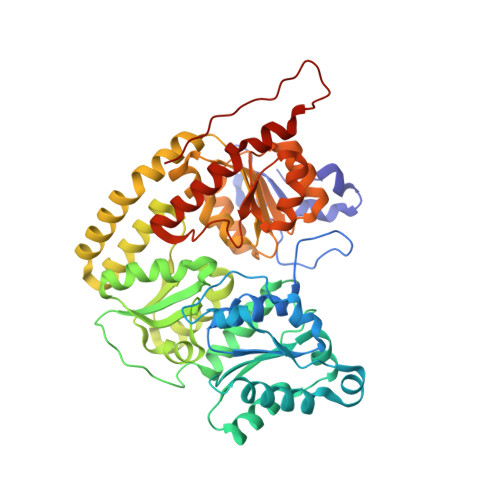
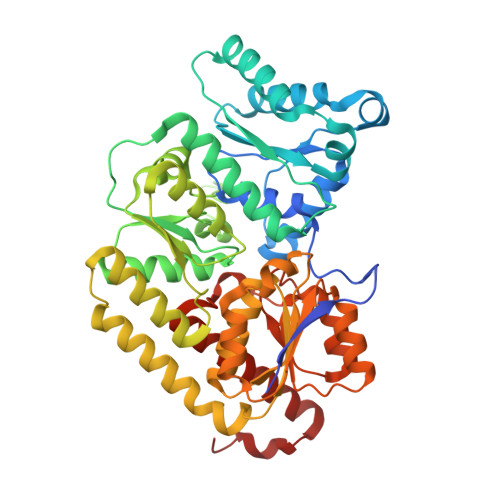
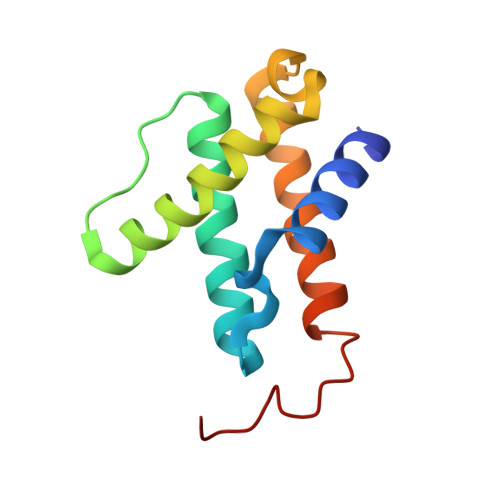
The structure of vanadium nitrogenase reveals an unusual bridging ligand.
Sippel, D., Einsle, O.(2017) Nat Chem Biol 13: 956-960
- PubMed: 28692069
- DOI: https://doi.org/10.1038/nchembio.2428
- Primary Citation of Related Structures:
5N6Y - PubMed Abstract:
Nitrogenases catalyze the reduction of dinitrogen (N 2 ) gas to ammonium at a complex heterometallic cofactor. This most commonly occurs at the FeMo cofactor (FeMoco), a [Mo-7Fe-9S-C] cluster whose exact reactivity and substrate-binding mode remain unknown. Alternative nitrogenases replace molybdenum with either vanadium or iron and differ in reactivity, most prominently in the ability of vanadium nitrogenase to reduce CO to hydrocarbons. Here we report the 1.35-Å structure of vanadium nitrogenase from Azotobacter vinelandii. The 240-kDa protein contains an additional α-helical subunit that is not present in molybdenum nitrogenase. The FeV cofactor (FeVco) is a [V-7Fe-8S-C] cluster with a homocitrate ligand to vanadium. Unexpectedly, it lacks one sulfide ion compared to FeMoco, which is replaced by a bridging ligand, likely a μ-1,3-carbonate. The anion fits into a pocket within the protein that is obstructed in molybdenum nitrogenase, and its different chemical character helps to rationalize the altered chemical properties of this unique N 2 - and CO-fixing enzyme.
- Lehrstuhl Biochemie, Institut für Biochemie, Albert-Ludwigs-Universität Freiburg, Freiburg Research Institute for Advanced Studies (FRIAS), and BIOSS Centre for Biological Signalling Studies, Freiburg, Germany.
Organizational Affiliation: