Crystal structure of truncated Aspartate Transcarbamoylase from Plasmodium falciparum
Lunev, S., Bosch, S.S., Batista, F.D.A., Wrenger, C., Groves, M.R.To be published.
Experimental Data Snapshot
Starting Model: other
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aspartate carbamoyltransferase | 385 | Plasmodium falciparum 3D7 | Mutation(s): 0 Gene Names: atcasE, MAL13P1.221 EC: 2.1.3.2 | 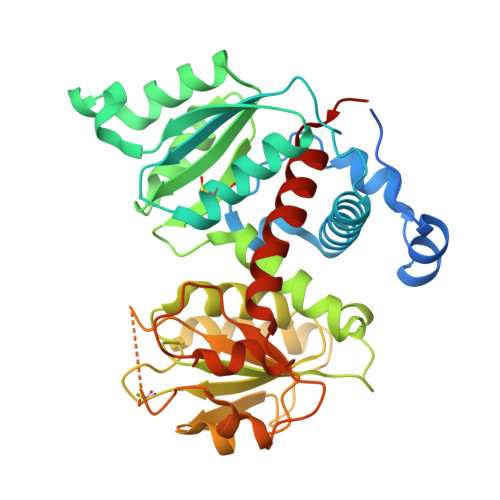 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FLC Query on FLC | H [auth C] | CITRATE ANION C6 H5 O7 KRKNYBCHXYNGOX-UHFFFAOYSA-K |  | ||
| PO4 Query on PO4 | E [auth A], G [auth B], K [auth C] | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| GOL Query on GOL | D [auth A], F [auth B], I [auth C], J [auth C] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSO Query on CSO | A, B, C | L-PEPTIDE LINKING | C3 H7 N O3 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 86.45 | α = 90 |
| b = 107.29 | β = 117.46 |
| c = 87.32 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| Coot | model building |
| Aimless | data scaling |
| MOLREP | phasing |