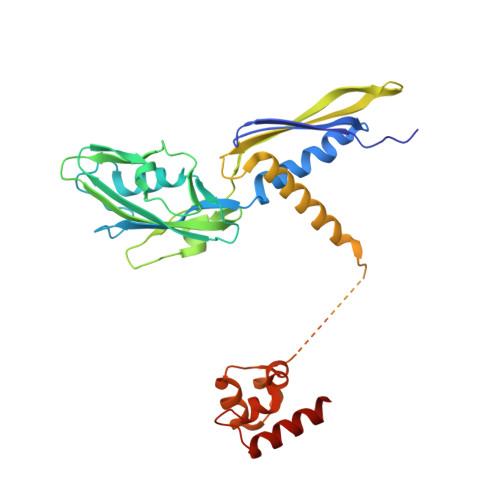
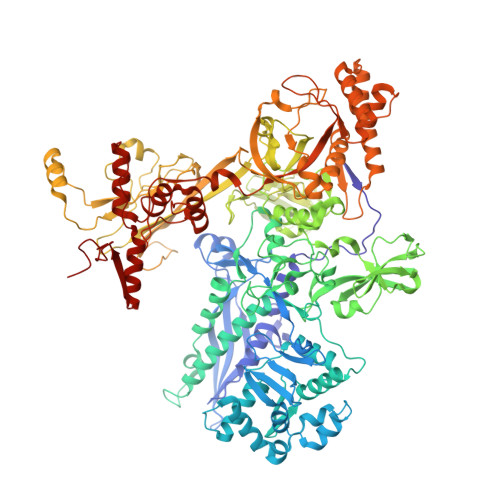
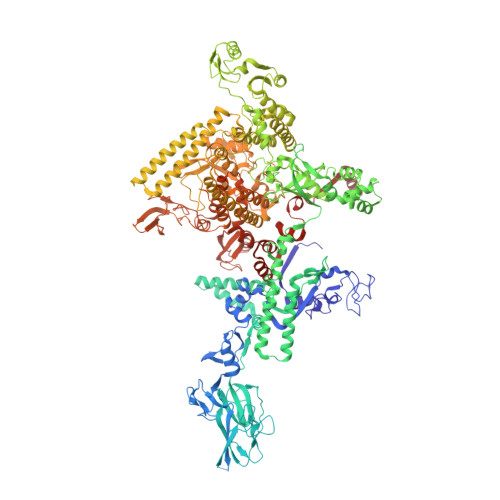
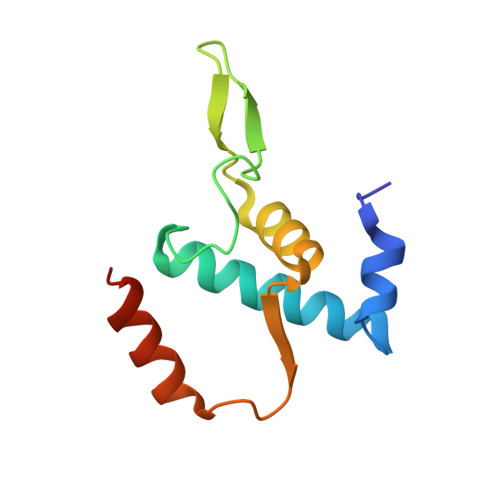
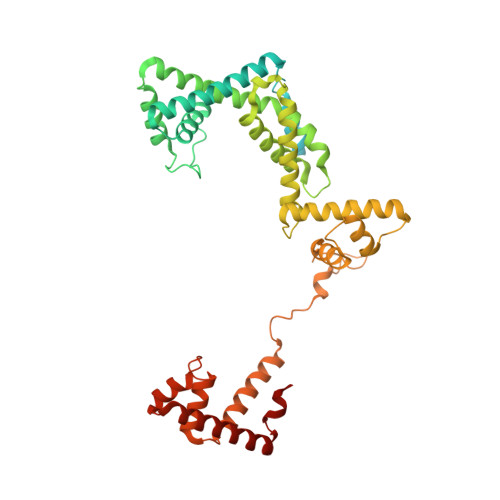
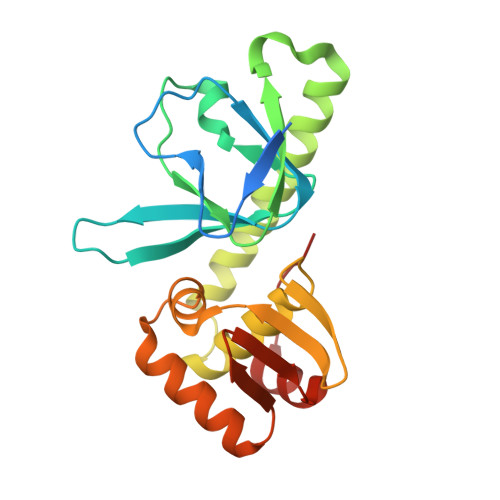
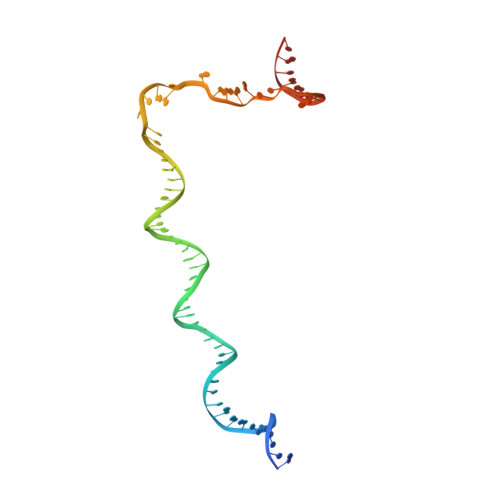
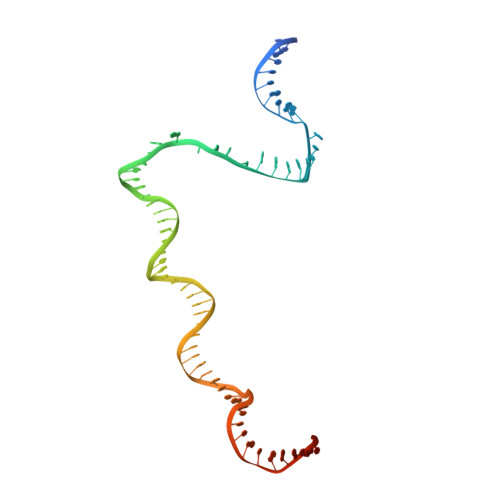
Structural basis of transcription activation.
Feng, Y., Zhang, Y., Ebright, R.H.(2016) Science 352: 1330-1333
- PubMed: 27284196
- DOI: https://doi.org/10.1126/science.aaf4417
- Primary Citation of Related Structures:
5I2D - PubMed Abstract:
Class II transcription activators function by binding to a DNA site overlapping a core promoter and stimulating isomerization of an initial RNA polymerase (RNAP)-promoter closed complex into a catalytically competent RNAP-promoter open complex. Here, we report a 4.4 angstrom crystal structure of an intact bacterial class II transcription activation complex. The structure comprises Thermus thermophilus transcription activator protein TTHB099 (TAP) [homolog of Escherichia coli catabolite activator protein (CAP)], T. thermophilus RNAP σ(A) holoenzyme, a class II TAP-dependent promoter, and a ribotetranucleotide primer. The structure reveals the interactions between RNAP holoenzyme and DNA responsible for transcription initiation and reveals the interactions between TAP and RNAP holoenzyme responsible for transcription activation. The structure indicates that TAP stimulates isomerization through simple, adhesive, stabilizing protein-protein interactions with RNAP holoenzyme.
- Waksman Institute and Department of Chemistry and Chemical Biology, Rutgers University, Piscataway, NJ 08854, USA.
Organizational Affiliation: