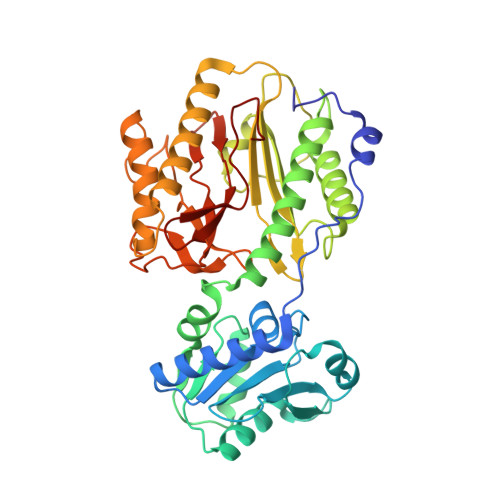
Crystal structure and biochemical investigations reveal novel mode of substrate selectivity and illuminate substrate inhibition and allostericity in a subfamily of Xaa-Pro dipeptidases.
Are, V.N., Kumar, A., Kumar, S., Goyal, V.D., Ghosh, B., Bhatnagar, D., Jamdar, S.N., Makde, R.D.(2017) Biochim Biophys Acta 1865: 153-164
- PubMed: 27816563
- DOI: https://doi.org/10.1016/j.bbapap.2016.10.016
- Primary Citation of Related Structures:
5FCF, 5FCH - PubMed Abstract:
Xaa-Pro dipeptidase (XPD) catalyzes hydrolysis of iminopeptide bond in dipeptides containing trans-proline as a second residue. XPDs are found in all living organisms and are believed to play an essential role in proline metabolism. Here, we report crystal structures and extensive enzymatic studies of XPD from Xanthomonas campestris (XPDxc), the first such comprehensive study of a bacterial XPD. We also report enzymatic activities of its ortholog from Mycobacterium tuberculosis (XPDmt). These enzymes are strictly dipeptidases with broad substrate specificities. They exhibit substrate inhibition and allostericity, as described earlier for XPD from Lactococcus lactis (XPDll). The structural, mutational and comparative data have revealed a novel mechanism of dipeptide selectivity and substrate binding in these enzymes. Moreover, we have identified conserved sequence motifs that distinguish these enzymes from other prolidases, thus defining a new subfamily. This study provides a suitable structural template for explaining unique properties of this XPDxc subfamily. In addition, we report unique structural features of XPDxc protein like an extended N-terminal tail region and absence of a conserved Tyr residue near the active site.
- High Pressure and Synchrotron Radiation Physics Division, Bhabha Atomic Research Centre, Mumbai, India; School of Biochemistry, Devi Ahilya Vishwavidyalaya, Indore, India.
Organizational Affiliation: