S. aureus MepR F27L Mutant bound to oligodeoxyribonucleotide
Birukou, I., Newman, C.E., Brennan, R.G.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| MarR family regulatory protein | 140 | Staphylococcus aureus | Mutation(s): 1 Gene Names: mepR | 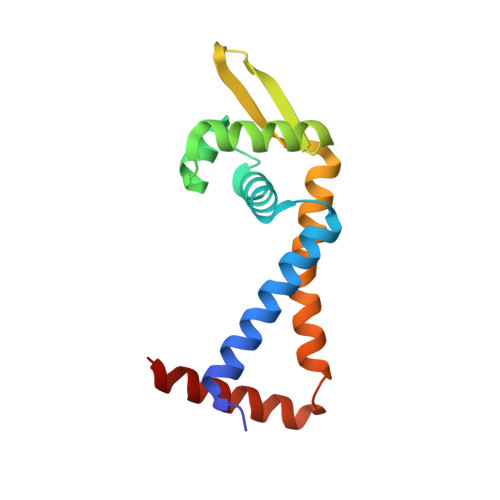 | |
UniProt | |||||
Find proteins for Q5Y812 (Staphylococcus aureus) Explore Q5Y812 Go to UniProtKB: Q5Y812 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5Y812 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (5'-D(*CP*GP*TP*TP*A)-3') | 5 | synthetic construct | 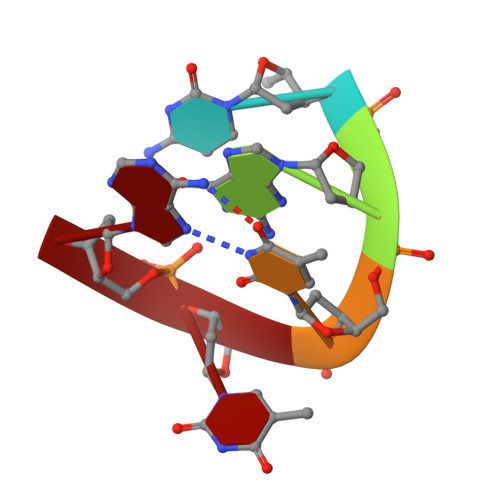 | ||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GOL Query on GOL | C [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 32.263 | α = 90 |
| b = 97.986 | β = 90 |
| c = 106.317 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| d*TREK | data reduction |
| d*TREK | data scaling |
| PHENIX | phasing |