Purification, crystallization and crystallographic analysis of the Pelota C terminal domian from human
Zhang, L., Cai, Q., Lin, T.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein pelota homolog | 121 | Homo sapiens | Mutation(s): 0 Gene Names: Pelota EC: 3.1 | 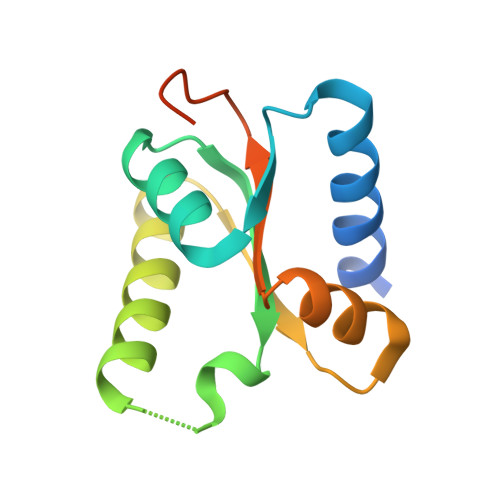 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9BRX2 (Homo sapiens) Explore Q9BRX2 Go to UniProtKB: Q9BRX2 | |||||
PHAROS: Q9BRX2 GTEx: ENSG00000152684 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9BRX2 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 78.822 | α = 90 |
| b = 78.822 | β = 90 |
| c = 197.456 | γ = 120 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| HKL-2000 | data scaling |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |