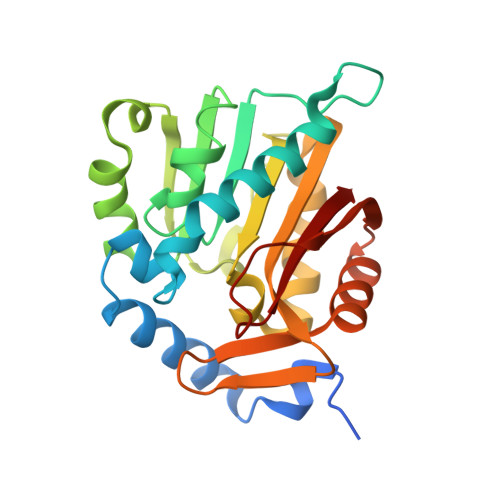
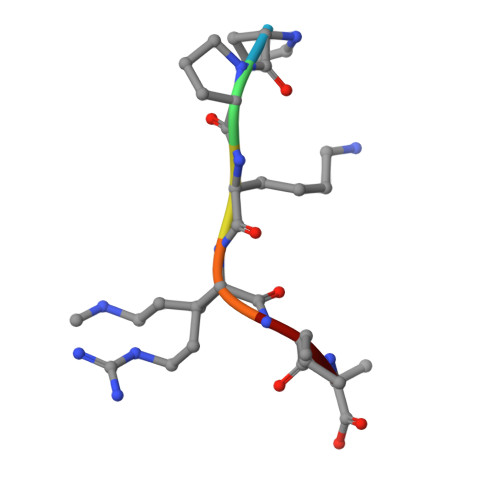
Structural basis for substrate recognition by the human N-terminal methyltransferase 1.
Dong, C., Mao, Y., Tempel, W., Qin, S., Li, L., Loppnau, P., Huang, R., Min, J.(2015) Genes Dev 29: 2343-2348
- PubMed: 26543161
- DOI: https://doi.org/10.1101/gad.270611.115
- Primary Citation of Related Structures:
5E1B, 5E1D, 5E1M, 5E1O, 5E2A, 5E2B - PubMed Abstract:
α-N-terminal methylation represents a highly conserved and prevalent post-translational modification, yet its biological function has remained largely speculative. The recent discovery of α-N-terminal methyltransferase 1 (NTMT1) and its physiological substrates propels the elucidation of a general role of α-N-terminal methylation in mediating DNA-binding ability of the modified proteins. The phenotypes, observed from both NTMT1 knockdown in breast cancer cell lines and knockout mouse models, suggest the potential involvement of α-N-terminal methylation in DNA damage response and cancer development. In this study, we report the first crystal structures of human NTMT1 in complex with cofactor S-adenosyl-L-homocysteine (SAH) and six substrate peptides, respectively, and reveal that NTMT1 contains two characteristic structural elements (a β hairpin and an N-terminal extension) that contribute to its substrate specificity. Our complex structures, coupled with mutagenesis, binding, and enzymatic studies, also present the key elements involved in locking the consensus substrate motif XPK (X indicates any residue type other than D/E) into the catalytic pocket for α-N-terminal methylation and explain why NTMT1 prefers an XPK sequence motif. We propose a catalytic mechanism for α-N-terminal methylation. Overall, this study gives us the first glimpse of the molecular mechanism of α-N-terminal methylation and potentially contributes to the advent of therapeutic agents for human diseases associated with deregulated α-N-terminal methylation.
- Structural Genomics Consortium, University of Toronto, Toronto, Ontaria M5G 1L7, Canada;
Organizational Affiliation: