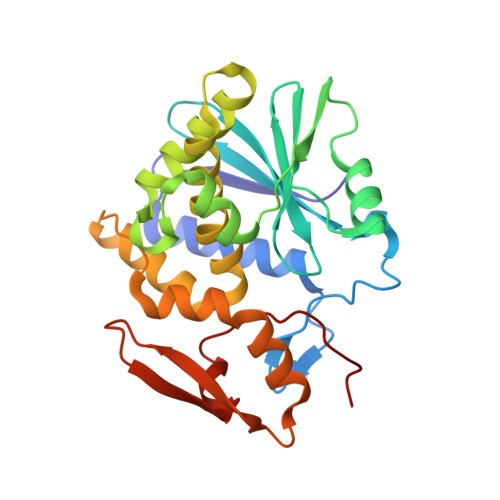
Structural analysis of nested neutralizing and non-neutralizing B cell epitopes on ricin toxin's enzymatic subunit.
Rudolph, M.J., Vance, D.J., Cassidy, M.S., Rong, Y., Shoemaker, C.B., Mantis, N.J.(2016) Proteins 84: 1162-1172
- PubMed: 27159829
- DOI: https://doi.org/10.1002/prot.25062
- Primary Citation of Related Structures:
4Z9K, 5E1H - PubMed Abstract:
In this report, we describe the X-ray crystal structures of two single domain camelid antibodies (VH H), F5 and F8, each in complex with ricin toxin's enzymatic subunit (RTA). F5 has potent toxin-neutralizing activity, while F8 has weak neutralizing activity. F5 buried a total of 1760 Å(2) in complex with RTA and made contact with three prominent secondary structural elements: α-helix B (Residues 98-106), β-strand h (Residues 113-117), and the C-terminus of α-helix D (Residues 154-156). F8 buried 1103 Å(2) in complex with RTA that was centered primarily on β-strand h. As such, the structural epitope of F8 is essentially nested within that of F5. All three of the F5 complementarity determining regions CDRs were involved in RTA contact, whereas F8 interactions were almost entirely mediated by CDR3, which essentially formed a seventh β-strand within RTA's centrally located β-sheet. A comparison of the two structures reported here to several previously reported (RTA-VH H) structures identifies putative contact sites on RTA, particularly α-helix B, associated with potent toxin-neutralizing activity. This information has implications for rational design of RTA-based subunit vaccines for biodefense. Proteins 2016; 84:1162-1172. © 2016 Wiley Periodicals, Inc.
- New York Structural Biology Center, New York, New York.
Organizational Affiliation: