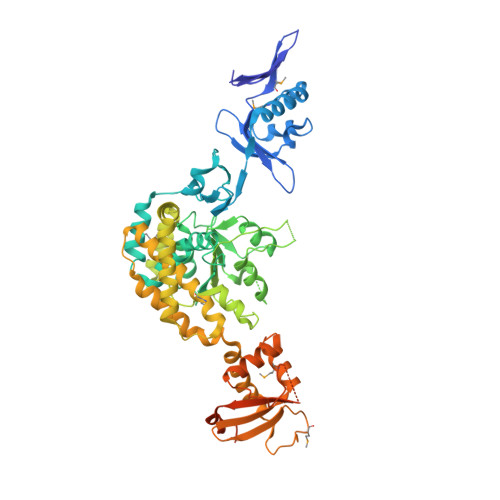
Staphylococcal SCCmec elements encode an active MCM-like helicase and thus may be replicative.
Mir-Sanchis, I., Roman, C.A., Misiura, A., Pigli, Y.Z., Boyle-Vavra, S., Rice, P.A.(2016) Nat Struct Mol Biol 23: 891-898
- PubMed: 27571176
- DOI: https://doi.org/10.1038/nsmb.3286
- Primary Citation of Related Structures:
5DGK - PubMed Abstract:
Methicillin-resistant Staphylococcus aureus (MRSA) is a public-health threat worldwide. Although the mobile genomic island responsible for this phenotype, staphylococcal cassette chromosome (SCC), has been thought to be nonreplicative, we predicted DNA-replication-related functions for some of the conserved proteins encoded by SCC. We show that one of these, Cch, is homologous to the self-loading initiator helicases of an unrelated family of genomic islands, that it is an active 3'-to-5' helicase and that the adjacent ORF encodes a single-stranded DNA-binding protein. Our 2.9-Å crystal structure of intact Cch shows that it forms a hexameric ring. Cch, like the archaeal and eukaryotic MCM-family replicative helicases, belongs to the pre-sensor II insert clade of AAA+ ATPases. Additionally, we found that SCC elements are part of a broader family of mobile elements, all of which encode a replication initiator upstream of their recombinases. Replication after excision would enhance the efficiency of horizontal gene transfer.
- Department of Biochemistry and Molecular Biology, The University of Chicago, Chicago, Illinois, USA.
Organizational Affiliation: