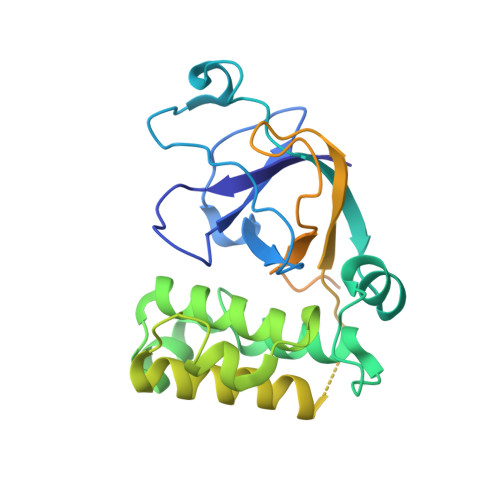
Structural and functional analysis of the fibronectin-binding protein FNE from Streptococcus equi spp. equi.
Tiouajni, M., Durand, D., Blondeau, K., Graille, M., Urvoas, A., Valerio-Lepiniec, M., Guellouz, A., Aumont-Nicaise, M., Minard, P., van Tilbeurgh, H.(2014) FEBS J 281: 5513-5531
- PubMed: 25290767
- DOI: https://doi.org/10.1111/febs.13092
- Primary Citation of Related Structures:
5DCQ - PubMed Abstract:
Streptococcus equi is a horse pathogen belonging to Lancefield group C. Infection by S. equi ssp. equi causes strangles, a serious and highly contagious disease of the upper respiratory tract. S. equi ssp. equi secretes a fibronectin (Fn)-binding protein, FNE, that does not contain cell wall-anchoring motifs. FNE binds to the gelatin-binding domain (GBD) of Fn, composed of the motifs (6) FI (12) FII (789) FI . FNE lacks the canonical Fn-binding peptide repeats observed in many microbial surface components recognizing adhesive matrix molecules. We found that the interaction between FNE and the human GBD is mediated by the binding of the disordered C-terminal region (residues 208-262) of FNE to the (789) FI GBD subfragment. The crystal structure of FNE showed that it is similar to the minor pilus protein Spy0125 of Streptococcus pyogenes, found at the end of pilus polymers and responsible for adhesion. FNE and Spy0125 both have a superimposable internal thioester bond between highly conserved Cys and Gln residues. Small-angle X-ray scattering of the FNE-(789) FI complex provided a model that aligns the C-terminal peptide of FNE with the E-strands of the FI domains, adopting the β-zipper extension model observed in previous structures of microbial surface components recognizing adhesive matrix molecule adhesion peptides bound to FI domains.
- Institut de Biochimie et de Biophysique Moléculaire et Cellulaire, UMR 8619 CNRS, Université Paris Sud, Orsay, France.
Organizational Affiliation: