An inhibitor of KDM5 demethylases reduces survival of drug-tolerant cancer cells.
Vinogradova, M., Gehling, V.S., Gustafson, A., Arora, S., Tindell, C.A., Wilson, C., Williamson, K.E., Guler, G.D., Gangurde, P., Manieri, W., Busby, J., Flynn, E.M., Lan, F., Kim, H.J., Odate, S., Cochran, A.G., Liu, Y., Wongchenko, M., Yang, Y., Cheung, T.K., Maile, T.M., Lau, T., Costa, M., Hegde, G.V., Jackson, E., Pitti, R., Arnott, D., Bailey, C., Bellon, S., Cummings, R.T., Albrecht, B.K., Harmange, J.C., Kiefer, J.R., Trojer, P., Classon, M.(2016) Nat Chem Biol 12: 531-538
- PubMed: 27214401
- DOI: https://doi.org/10.1038/nchembio.2085
- Primary Citation of Related Structures:
5CEH - PubMed Abstract:
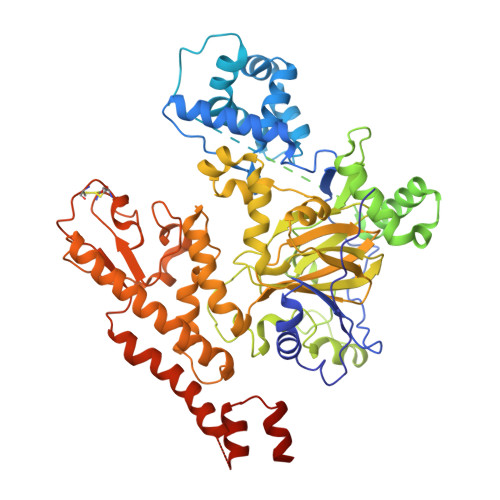
The KDM5 family of histone demethylases catalyzes the demethylation of histone H3 on lysine 4 (H3K4) and is required for the survival of drug-tolerant persister cancer cells (DTPs). Here we report the discovery and characterization of the specific KDM5 inhibitor CPI-455. The crystal structure of KDM5A revealed the mechanism of inhibition of CPI-455 as well as the topological arrangements of protein domains that influence substrate binding. CPI-455 mediated KDM5 inhibition, elevated global levels of H3K4 trimethylation (H3K4me3) and decreased the number of DTPs in multiple cancer cell line models treated with standard chemotherapy or targeted agents. These findings show that pretreatment of cancer cells with a KDM5-specific inhibitor results in the ablation of a subpopulation of cancer cells that can serve as the founders for therapeutic relapse.
- Genentech Inc., South San Francisco, California, USA.
Organizational Affiliation: