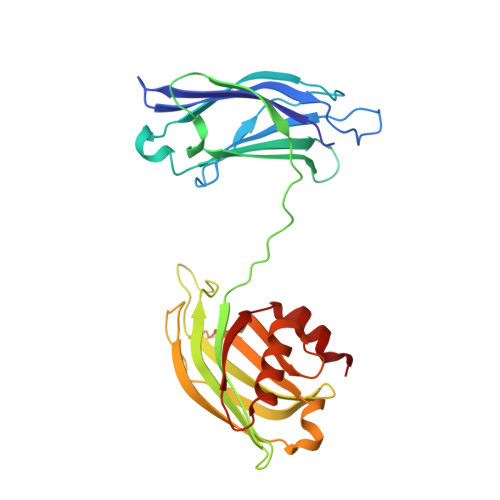
Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis.
Zhou, Q., Lai, Y., Bacaj, T., Zhao, M., Lyubimov, A.Y., Uervirojnangkoorn, M., Zeldin, O.B., Brewster, A.S., Sauter, N.K., Cohen, A.E., Soltis, S.M., Alonso-Mori, R., Chollet, M., Lemke, H.T., Pfuetzner, R.A., Choi, U.B., Weis, W.I., Diao, J., Sudhof, T.C., Brunger, A.T.(2015) Nature 525: 62-67
- PubMed: 26280336
- DOI: https://doi.org/10.1038/nature14975
- Primary Citation of Related Structures:
5CCG, 5CCH, 5CCI, 5CCJ - PubMed Abstract:
Synaptotagmin-1 and neuronal SNARE proteins have central roles in evoked synchronous neurotransmitter release; however, it is unknown how they cooperate to trigger synaptic vesicle fusion. Here we report atomic-resolution crystal structures of Ca(2+)- and Mg(2+)-bound complexes between synaptotagmin-1 and the neuronal SNARE complex, one of which was determined with diffraction data from an X-ray free-electron laser, leading to an atomic-resolution structure with accurate rotamer assignments for many side chains. The structures reveal several interfaces, including a large, specific, Ca(2+)-independent and conserved interface. Tests of this interface by mutagenesis suggest that it is essential for Ca(2+)-triggered neurotransmitter release in mouse hippocampal neuronal synapses and for Ca(2+)-triggered vesicle fusion in a reconstituted system. We propose that this interface forms before Ca(2+) triggering, moves en bloc as Ca(2+) influx promotes the interactions between synaptotagmin-1 and the plasma membrane, and consequently remodels the membrane to promote fusion, possibly in conjunction with other interfaces.
- Department of Molecular and Cellular Physiology, Howard Hughes Medical Institute, Stanford University, Stanford, California 94305, USA.
Organizational Affiliation: