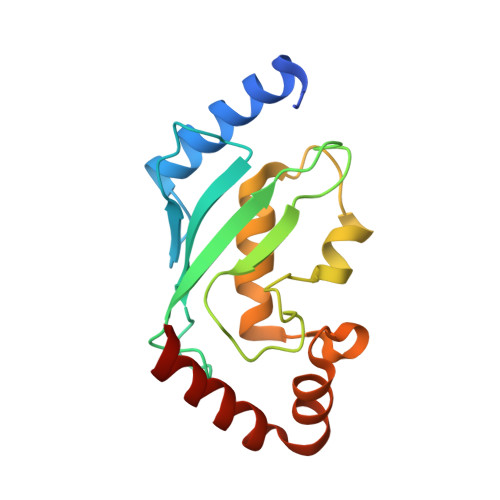
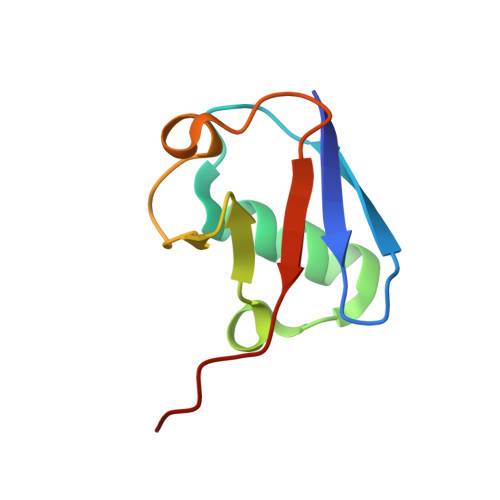
Crystal Structure of a Ube2S-Ubiquitin Conjugate.
Lorenz, S., Bhattacharyya, M., Feiler, C., Rape, M., Kuriyan, J.(2016) PLoS One 11: e0147550-e0147550
- PubMed: 26828794
- DOI: https://doi.org/10.1371/journal.pone.0147550
- Primary Citation of Related Structures:
5BNB - PubMed Abstract:
Protein ubiquitination occurs through the sequential formation and reorganization of specific protein-protein interfaces. Ubiquitin-conjugating (E2) enzymes, such as Ube2S, catalyze the formation of an isopeptide linkage between the C-terminus of a "donor" ubiquitin and a primary amino group of an "acceptor" ubiquitin molecule. This reaction involves an intermediate, in which the C-terminus of the donor ubiquitin is thioester-bound to the active site cysteine of the E2 and a functionally important interface is formed between the two proteins. A docked model of a Ube2S-donor ubiquitin complex was generated previously, based on chemical shift mapping by NMR, and predicted contacts were validated in functional studies. We now present the crystal structure of a covalent Ube2S-ubiquitin complex. The structure contains an interface between Ube2S and ubiquitin in trans that resembles the earlier model in general terms, but differs in detail. The crystallographic interface is more hydrophobic than the earlier model and is stable in molecular dynamics (MD) simulations. Remarkably, the docked Ube2S-donor complex converges readily to the configuration seen in the crystal structure in 3 out of 8 MD trajectories. Since the crystallographic interface is fully consistent with mutational effects, this indicates that the structure provides an energetically favorable representation of the functionally critical Ube2S-donor interface.
- California Institute for Quantitative Biosciences, University of California, Berkeley, California, United States of America.
Organizational Affiliation: