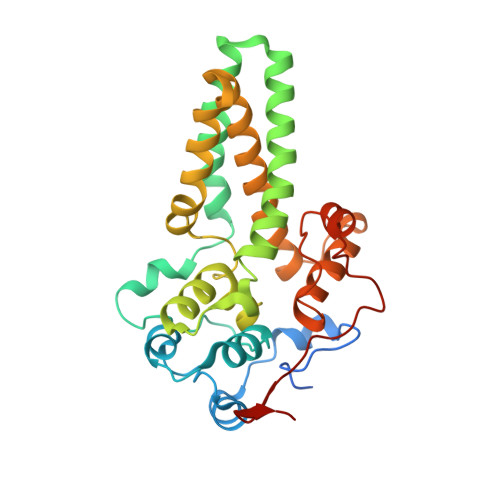
The Crystal Structure of an Integral Membrane Fatty Acid alpha-Hydroxylase.
Zhu, G., Koszelak-Rosenblum, M., Connelly, S.M., Dumont, M.E., Malkowski, M.G.(2015) J Biological Chem 290: 29820-29833
- PubMed: 26515067
- DOI: https://doi.org/10.1074/jbc.M115.680124
- Primary Citation of Related Structures:
4ZR0, 4ZR1 - PubMed Abstract:
Neuronal electrical impulse propagation is facilitated by the myelin sheath, a compact membrane surrounding the axon. The myelin sheath is highly enriched in galactosylceramide (GalCer) and its sulfated derivative sulfatide. Over 50% of GalCer and sulfatide in myelin is hydroxylated by the integral membrane enzyme fatty acid 2-hydroxylase (FA2H). GalCer hydroxylation contributes to the compact nature of the myelin membrane, and mutations in FA2H result in debilitating leukodystrophies and spastic paraparesis. We report here the 2.6 Å crystal structure of sphingolipid α-hydroxylase (Scs7p), a yeast homolog of FA2H. The Scs7p core is composed of a helical catalytic cap domain that sits atop four transmembrane helices that anchor the enzyme in the endoplasmic reticulum. The structure contains two zinc atoms coordinated by the side chains of 10 highly conserved histidines within a dimetal center located near the plane of the cytosolic membrane. We used a yeast genetic approach to confirm the important role of the dimetal-binding histidines in catalysis and identified Tyr-322 and Asp-323 as critical determinants involved in the hydroxylase reaction. Examination of the Scs7p structure, coupled with molecular dynamics simulations, allowed for the generation of a model of ceramide binding to Scs7p. Comparison of the Scs7p structure and substrate-binding model to the structure of steroyl-CoA desaturase revealed significant differences in the architecture of the catalytic cap domain and location of the dimetal centers with respect to the membrane. These observations provide insight into the different mechanisms of substrate binding and recognition of substrates by the hydroxylase and desaturase enzymes.
- From the Hauptman-Woodward Medical Research Institute, Buffalo, New York 14203.
Organizational Affiliation: