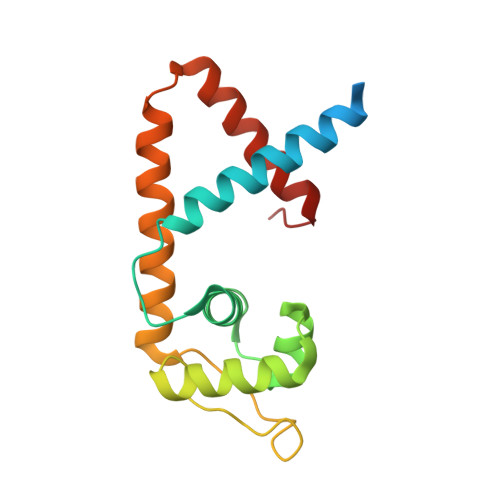
Structure of the MarR family protein Rv0880 from Mycobacterium tuberculosis.
Gao, Y.R., Feng, N., Chen, T., Li, D.F., Bi, L.J.(2015) Acta Crystallogr F Struct Biol Commun 71: 741-745
- PubMed: 26057805
- DOI: https://doi.org/10.1107/S2053230X15007281
- Primary Citation of Related Structures:
4YIF - PubMed Abstract:
Rv0880 from the pathogen Mycobacterium tuberculosis is classified as a MarR family protein in the Pfam database. It consists of 143 amino acids and has an isoelectric point of 10.9. Crystals of Rv0880 belonged to space group P1, with unit-cell parameters a = 54.97, b = 69.60, c = 70.32 Å, α = 103.71, β = 111.06, γ = 105.83°. The structure of the MarR family transcription regulator Rv0880 was solved at a resolution of 2.0 Å with an R(cryst) and R(free) of 21.2 and 24.9%, respectively. The dimeric structure resembles that of other MarR proteins, with each subunit comprising a winged helix-turn-helix domain connected to an α-helical dimerization domain.
- Key Laboratory of RNA Biology, National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, People's Republic of China.
Organizational Affiliation: