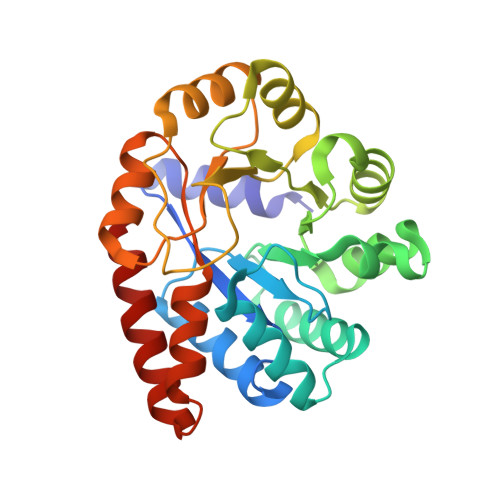
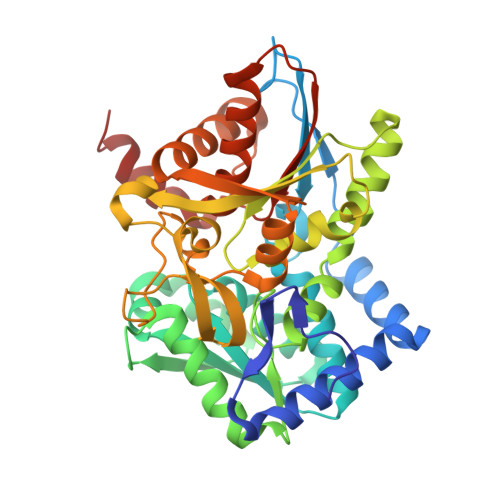
Crystal structure of Tryptophan Synthase from Salmonella typhimurium in complex with 2-({[4-(Trifluoromethoxy)Phenyl]Sulfonyl}Amino)Ethyl Dihydrogen Phosphate (F9F) inhibitor in the alpha site and ammonium ion in the metal coordination site.
Hilario, E., Caulkins, B.G., Young, R.P., Niks, D., Dunn, M.F., Mueller, L.J., Fan, L.To be published.