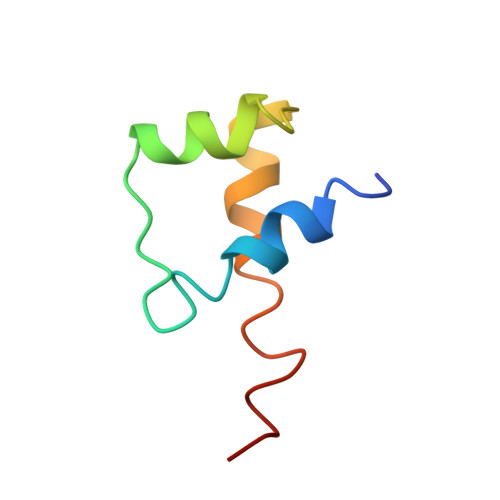
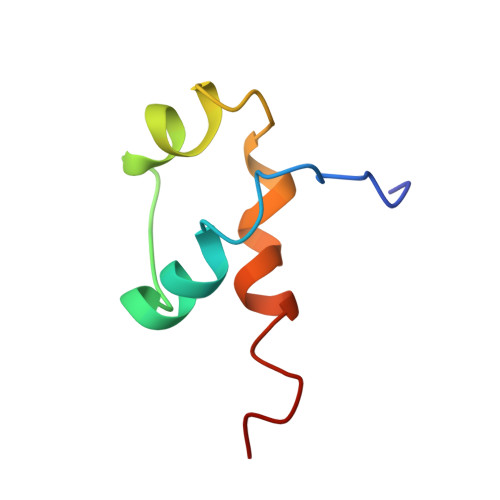
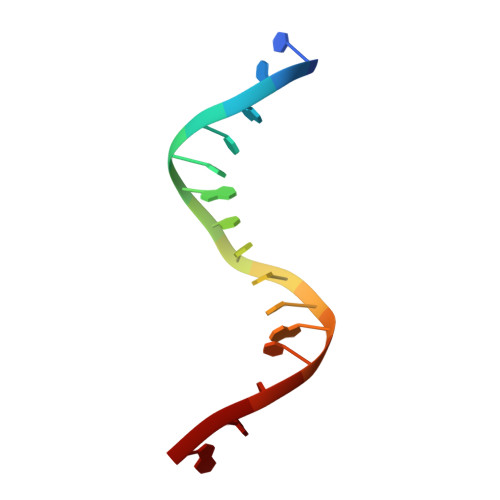
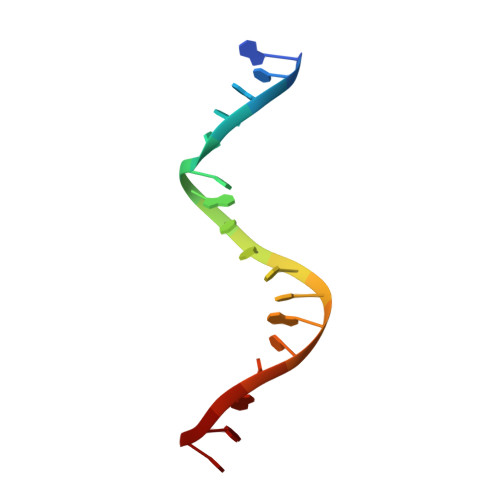
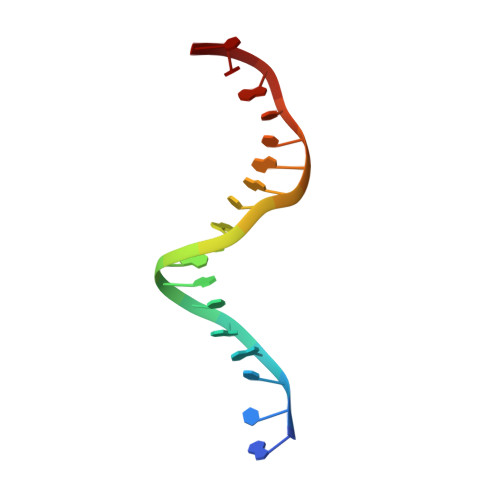
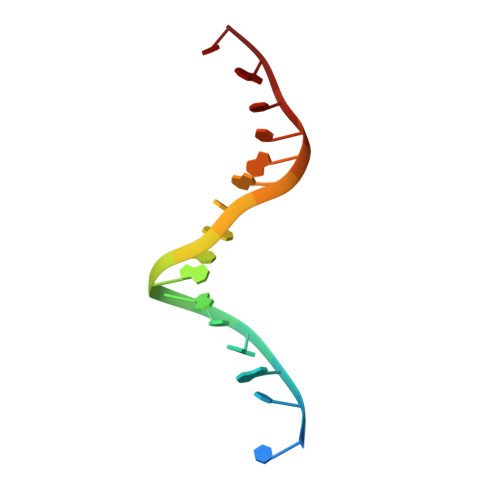
DNA-dependent formation of transcription factor pairs alters their binding specificity.
Jolma, A., Yin, Y., Nitta, K.R., Dave, K., Popov, A., Taipale, M., Enge, M., Kivioja, T., Morgunova, E., Taipale, J.(2015) Nature 527: 384-388
- PubMed: 26550823
- DOI: https://doi.org/10.1038/nature15518
- Primary Citation of Related Structures:
4XRM, 4XRS, 5BNG - PubMed Abstract:
Gene expression is regulated by transcription factors (TFs), proteins that recognize short DNA sequence motifs. Such sequences are very common in the human genome, and an important determinant of the specificity of gene expression is the cooperative binding of multiple TFs to closely located motifs. However, interactions between DNA-bound TFs have not been systematically characterized. To identify TF pairs that bind cooperatively to DNA, and to characterize their spacing and orientation preferences, we have performed consecutive affinity-purification systematic evolution of ligands by exponential enrichment (CAP-SELEX) analysis of 9,400 TF-TF-DNA interactions. This analysis revealed 315 TF-TF interactions recognizing 618 heterodimeric motifs, most of which have not been previously described. The observed cooperativity occurred promiscuously between TFs from diverse structural families. Structural analysis of the TF pairs, including a novel crystal structure of MEIS1 and DLX3 bound to their identified recognition site, revealed that the interactions between the TFs were predominantly mediated by DNA. Most TF pair sites identified involved a large overlap between individual TF recognition motifs, and resulted in recognition of composite sites that were markedly different from the individual TF's motifs. Together, our results indicate that the DNA molecule commonly plays an active role in cooperative interactions that define the gene regulatory lexicon.
- Department of Biosciences and Nutrition, Karolinska Institutet, SE 141 83, Sweden.
Organizational Affiliation: