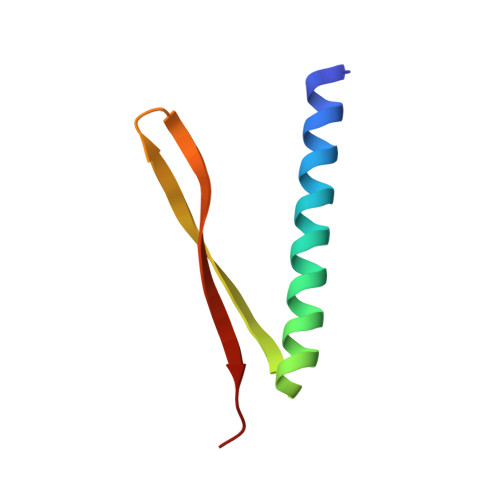
Crystal structure of GnsA from Escherichia coli
Wei, Y., Zhan, L., Gao, Z., Prive, G.G., Dong, Y.(2015) Biochem Biophys Res Commun 462: 1-7
- PubMed: 25839658
- DOI: https://doi.org/10.1016/j.bbrc.2015.03.133
- Primary Citation of Related Structures:
4XO1, 4XO2 - PubMed Abstract:
Escherichia Coli GnsA is a regulator of phosphatidylethanolamine synthesis and functions as a suppressor of both a secG null mutation and fabA6 mutations. GnsA may also be a toxin with the cognate antitoxin YmcE. Here we report the crystal structure of GnsA to 1.8 Å. GnsA forms a V shaped hairpin structure that is tightly associated into a homodimer. Our comprehensive structural study suggests that GnsA is structurally similar to an outer membrane protein, suggesting a function of protein binding.
- School of Life Science, University of Science and Technology of China, Hefei 230027, China; Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada. Electronic address: yongwei@uhnresearch.ca.
Organizational Affiliation: