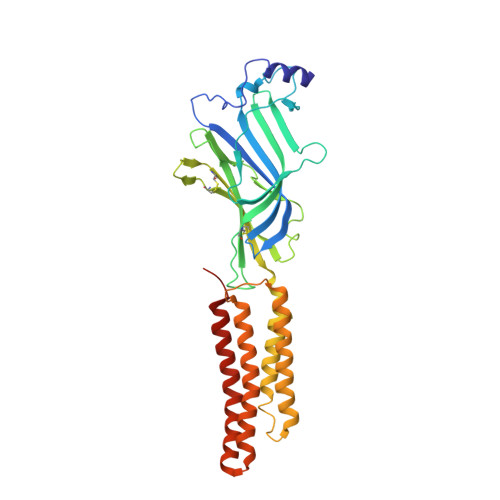
X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors.
Althoff, T., Hibbs, R.E., Banerjee, S., Gouaux, E.(2014) Nature 512: 333-337
- PubMed: 25143115
- DOI: https://doi.org/10.1038/nature13669
- Primary Citation of Related Structures:
4TNV, 4TNW - PubMed Abstract:
Cys-loop receptors are neurotransmitter-gated ion channels that are essential mediators of fast chemical neurotransmission and are associated with a large number of neurological diseases and disorders, as well as parasitic infections. Members of this ion channel superfamily mediate excitatory or inhibitory neurotransmission depending on their ligand and ion selectivity. Structural information for Cys-loop receptors comes from several sources including electron microscopic studies of the nicotinic acetylcholine receptor, high-resolution X-ray structures of extracellular domains and X-ray structures of bacterial orthologues. In 2011 our group published structures of the Caenorhabditis elegans glutamate-gated chloride channel (GluCl) in complex with the allosteric partial agonist ivermectin, which provided insights into the structure of a possibly open state of a eukaryotic Cys-loop receptor, the basis for anion selectivity and channel block, and the mechanism by which ivermectin and related molecules stabilize the open state and potentiate neurotransmitter binding. However, there remain unanswered questions about the mechanism of channel opening and closing, the location and nature of the shut ion channel gate, the transitions between the closed/resting, open/activated and closed/desensitized states, and the mechanism by which conformational changes are coupled between the extracellular, orthosteric agonist binding domain and the transmembrane, ion channel domain. Here we present two conformationally distinct structures of C. elegans GluCl in the absence of ivermectin. Structural comparisons reveal a quaternary activation mechanism arising from rigid-body movements between the extracellular and transmembrane domains and a mechanism for modulation of the receptor by phospholipids.
- 1] Vollum Institute, Oregon Health &Science University, 3181 SW Sam Jackson Park Road, Portland, Oregon 97239, USA [2] Department of Physiology, David Geffen School of Medicine, University of California Los Angeles, 10833 Le Conte Avenue, Los Angeles, California 90095-1751, USA (T.A.); Department of Neuroscience, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas 75390-9111, USA (R.E.H.). [3].
Organizational Affiliation: