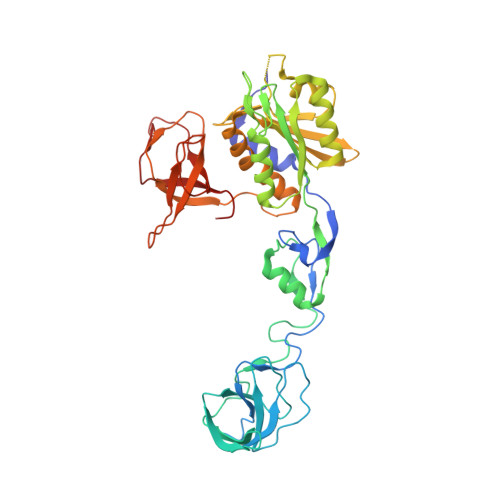
Molecular basis of the alternative recruitment of GABAA versus glycine receptors through gephyrin.
Maric, H.M., Kasaragod, V.B., Hausrat, T.J., Kneussel, M., Tretter, V., Strmgaard, K., Schindelin, H.(2014) Nat Commun 5: 5767-5767
- PubMed: 25531214
- DOI: https://doi.org/10.1038/ncomms6767
- Primary Citation of Related Structures:
4TK1, 4TK2, 4TK3, 4TK4 - PubMed Abstract:
γ-Aminobutyric acid type A and glycine receptors (GABA(A)Rs, GlyRs) are the major inhibitory neurotransmitter receptors and contribute to many synaptic functions, dysfunctions and human diseases. GABA(A)Rs are important drug targets regulated by direct interactions with the scaffolding protein gephyrin. Here we deduce the molecular basis of this interaction by chemical, biophysical and structural studies of the gephyrin-GABA(A)R α3 complex, revealing that the N-terminal region of the α3 peptide occupies the same binding site as the GlyR β subunit, whereas the C-terminal moiety, which is conserved among all synaptic GABA(A)R α subunits, engages in unique interactions. Thermodynamic dissections of the gephyrin-receptor interactions identify two residues as primary determinants for gephyrin's subunit preference. This first structural evidence for the gephyrin-mediated synaptic accumulation of GABA(A)Rs offers a framework for future investigations into the regulation of inhibitory synaptic strength and for the development of mechanistically and therapeutically relevant compounds targeting the gephyrin-GABA(A)R interaction.
- 1] Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Josef-Schneider-Straße 2, Building D15, D-97080 Würzburg, Germany [2] Department of Drug Design and Pharmacology, University of Copenhagen, Universitetsparken 2, DK-2100 Copenhagen, Denmark.
Organizational Affiliation: