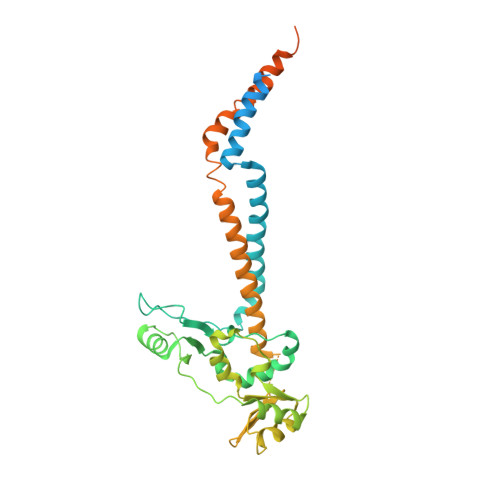
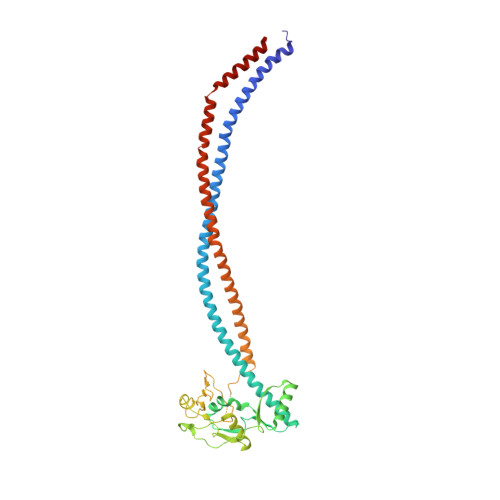
Molecular Basis for SMC Rod Formation and Its Dissolution upon DNA Binding.
Soh, Y.M., Burmann, F., Shin, H.C., Oda, T., Jin, K.S., Toseland, C.P., Kim, C., Lee, H., Kim, S.J., Kong, M.S., Durand-Diebold, M.L., Kim, Y.G., Kim, H.M., Lee, N.K., Sato, M., Oh, B.H., Gruber, S.(2015) Mol Cell 57: 290-303
- PubMed: 25557547
- DOI: https://doi.org/10.1016/j.molcel.2014.11.023
- Primary Citation of Related Structures:
4RSI, 4RSJ - PubMed Abstract:
SMC condensin complexes are central modulators of chromosome superstructure in all branches of life. Their SMC subunits form a long intramolecular coiled coil, which connects a constitutive "hinge" dimerization domain with an ATP-regulated "head" dimerization module. Here, we address the structural arrangement of the long coiled coils in SMC complexes. We unequivocally show that prokaryotic Smc-ScpAB, eukaryotic condensin, and possibly also cohesin form rod-like structures, with their coiled coils being closely juxtaposed and accurately anchored to the hinge. Upon ATP-induced binding of DNA to the hinge, however, Smc switches to a more open configuration. Our data suggest that a long-distance structural transition is transmitted from the Smc head domains to regulate Smc-ScpAB's association with DNA. These findings uncover a conserved architectural theme in SMC complexes, provide a mechanistic basis for Smc's dynamic engagement with chromosomes, and offer a molecular explanation for defects in Cornelia de Lange syndrome.
- Department of Biological Sciences, KAIST Institute for the Biocentury, Cancer Metastasis Control Center, Korea Advanced Institute of Science and Technology, Daejeon 305-701, Korea.
Organizational Affiliation: