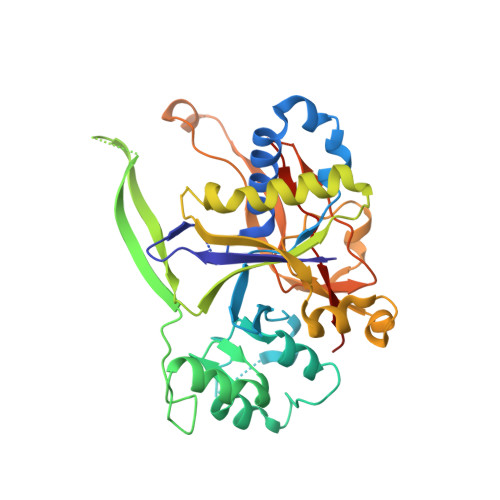
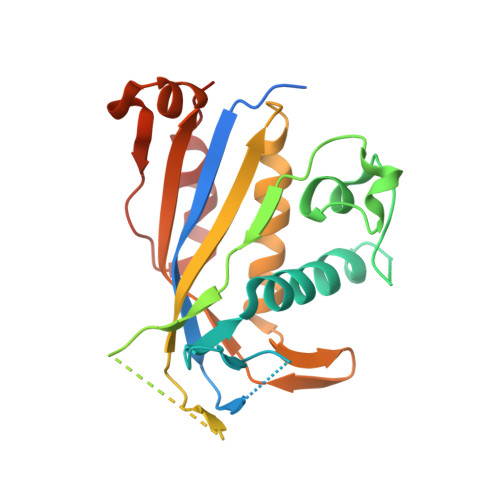
Crystal structure of the Csm3-Csm4 subcomplex in the type III-A CRISPR-Cas interference complex.
Numata, T., Inanaga, H., Sato, C., Osawa, T.(2015) J Mol Biology 427: 259-273
- PubMed: 25451598
- DOI: https://doi.org/10.1016/j.jmb.2014.09.029
- Primary Citation of Related Structures:
4QTS - PubMed Abstract:
Clustered, regularly interspaced, short palindromic repeat (CRISPR) loci play a pivotal role in the prokaryotic host defense system against invading genetic materials. The CRISPR loci are transcribed to produce CRISPR RNAs (crRNAs), which form interference complexes with CRISPR-associated (Cas) proteins to target the invading nucleic acid for degradation. The interference complex of the type III-A CRISPR-Cas system is composed of five Cas proteins (Csm1-Csm5) and a crRNA, and targets invading DNA. Here, we show that the Csm1, Csm3, and Csm4 proteins from Methanocaldococcus jannaschii form a stable subcomplex. We also report the crystal structure of the M. jannaschii Csm3-Csm4 subcomplex at 3.1Å resolution. The complex structure revealed the presence of a basic concave surface around their interface, suggesting the RNA and/or DNA binding ability of the complex. A gel retardation analysis showed that the Csm3-Csm4 complex binds single-stranded RNA in a non-sequence-specific manner. Csm4 structurally resembles Cmr3, a component of the type III-B CRISPR-Cas interference complex. Based on bioinformatics, we constructed a model structure of the Csm1-Csm4-Csm3 ternary complex, which provides insights into its role in the Csm interference complex.
- Biomedical Research Institute, National Institute of Advanced Industrial Science and Technology, 1-1-1 Higashi, Tsukuba-shi, Ibaraki 305-8566, Japan. Electronic address: t-numata@aist.go.jp.
Organizational Affiliation: