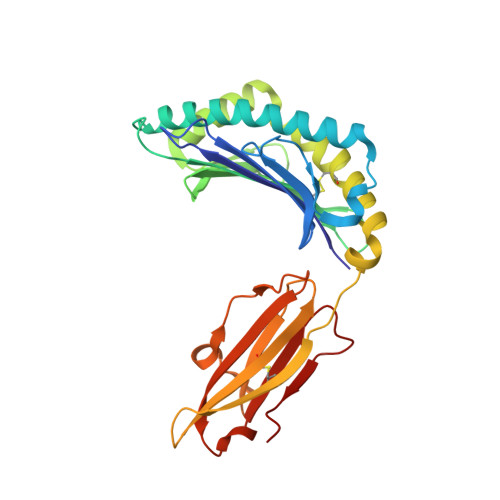
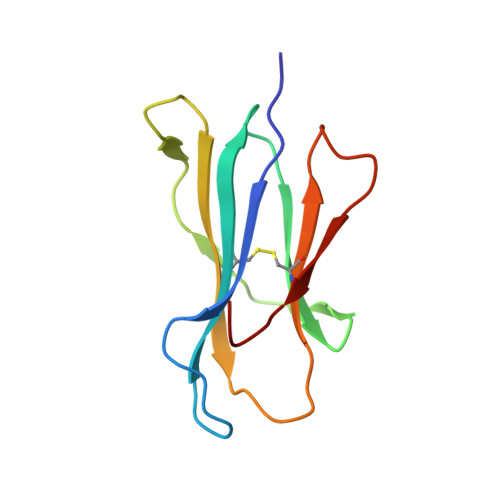
A Molecular Basis for the Interplay between T Cells, Viral Mutants, and Human Leukocyte Antigen Micropolymorphism.
Liu, Y.C., Chen, Z., Neller, M.A., Miles, J.J., Purcell, A.W., McCluskey, J., Burrows, S.R., Rossjohn, J., Gras, S.(2014) J Biological Chem 289: 16688-16698
- PubMed: 24759101
- DOI: https://doi.org/10.1074/jbc.M114.563502
- Primary Citation of Related Structures:
4PR5, 4PRA, 4PRB, 4PRD, 4PRE, 4PRH, 4PRI, 4PRN, 4PRP - PubMed Abstract:
Mutations within T cell epitopes represent a common mechanism of viral escape from the host protective immune response. The diverse T cell repertoire and the extensive human leukocyte antigen (HLA) polymorphism across populations is the evolutionary response to viral mutation. However, the molecular basis underpinning the interplay between HLA polymorphism, the T cell repertoire, and viral escape is unclear. Here we investigate the T cell response to a HLA-B*35:01- and HLA-B*35:08-restricted (407)HPVGEADYFEY(417) epitope from Epstein-Barr virus and naturally occurring variants at positions 4 and 5 thereof. Each viral variant differently impacted on the epitope's flexibility and conformation when bound to HLA-B*35:08 or HLA-B*35:01. We provide a molecular basis for understanding how the single residue polymorphism that discriminates between HLA-B*35:01/08 profoundly impacts on T cell receptor recognition. Surprisingly, one viral variant (P5-Glu to P5-Asp) effectively changed restriction preference from HLA-B*35:01 to HLA-B*35:08. Collectively, our study portrays the interplay between the T cell response, viral escape, and HLA polymorphism, whereby HLA polymorphism enables altered presentation of epitopes from different strains of Epstein-Barr virus.
- From the Department of Biochemistry and Molecular Biology, School of Biomedical Sciences, Monash University, Clayton, Melbourne 3800, Australia.
Organizational Affiliation: