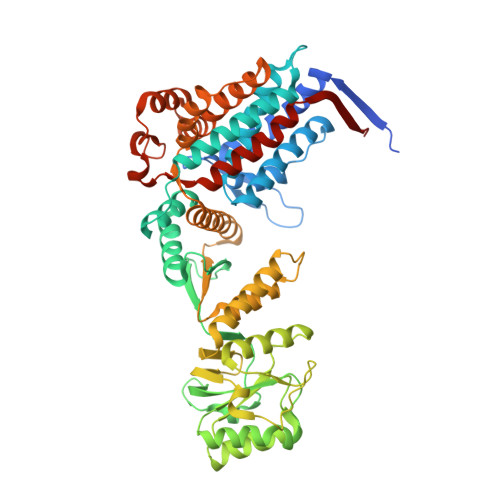
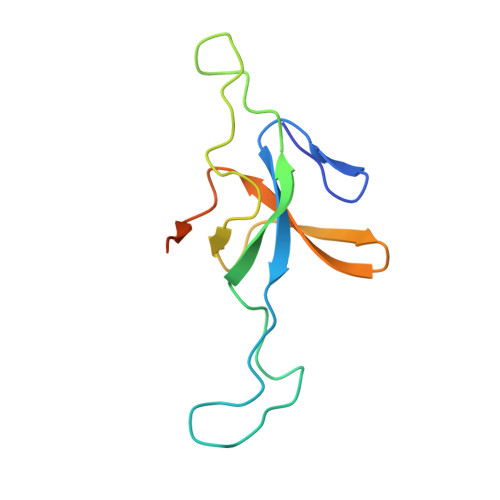
Crystal structure of the human mitochondrial chaperonin symmetrical football complex.
Nisemblat, S., Yaniv, O., Parnas, A., Frolow, F., Azem, A.(2015) Proc Natl Acad Sci U S A 112: 6044-6049
- PubMed: 25918392
- DOI: https://doi.org/10.1073/pnas.1411718112
- Primary Citation of Related Structures:
4PJ1 - PubMed Abstract:
Human mitochondria harbor a single type I chaperonin system that is generally thought to function via a unique single-ring intermediate. To date, no crystal structure has been published for any mammalian type I chaperonin complex. In this study, we describe the crystal structure of a football-shaped, double-ring human mitochondrial chaperonin complex at 3.15 Å, which is a novel intermediate, likely representing the complex in an early stage of dissociation. Interestingly, the mitochondrial chaperonin was captured in a state that exhibits subunit asymmetry within the rings and nucleotide symmetry between the rings. Moreover, the chaperonin tetradecamers show a different interring subunit arrangement when compared to GroEL. Our findings suggest that the mitochondrial chaperonins use a mechanism that is distinct from the mechanism of the well-studied Escherichia coli system.
- Departments of Biochemistry and Molecular Biology and The Daniella Rich Institute for Structural Biology, The George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv 69978, Israel.
Organizational Affiliation: