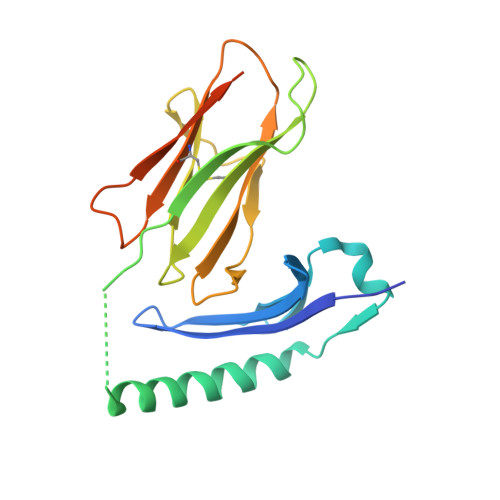
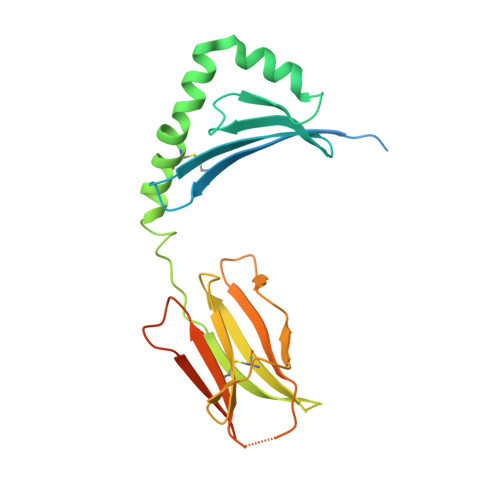
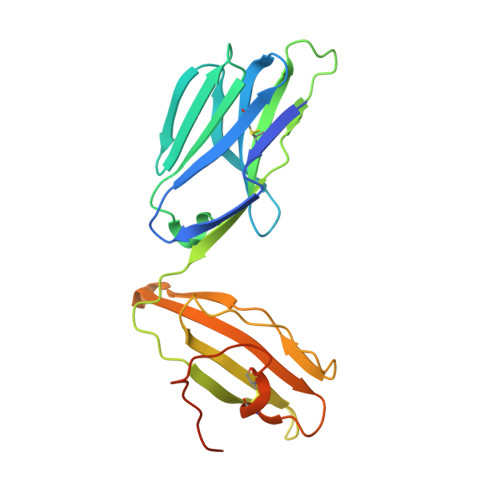
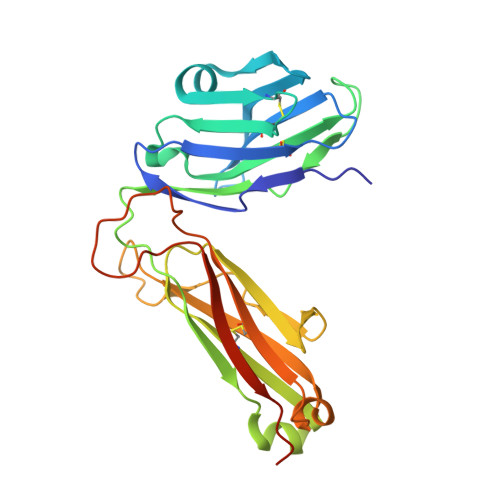
Deconstructing the Peptide-MHC Specificity of T Cell Recognition.
Birnbaum, M.E., Mendoza, J.L., Sethi, D.K., Dong, S., Glanville, J., Dobbins, J., Ozkan, E., Davis, M.M., Wucherpfennig, K.W., Garcia, K.C.(2014) Cell 157: 1073-1087
- PubMed: 24855945
- DOI: https://doi.org/10.1016/j.cell.2014.03.047
- Primary Citation of Related Structures:
4P2O, 4P2Q, 4P2R - PubMed Abstract:
In order to survey a universe of major histocompatibility complex (MHC)-presented peptide antigens whose numbers greatly exceed the diversity of the T cell repertoire, T cell receptors (TCRs) are thought to be cross-reactive. However, the nature and extent of TCR cross-reactivity has not been conclusively measured experimentally. We developed a system to identify MHC-presented peptide ligands by combining TCR selection of highly diverse yeast-displayed peptide-MHC libraries with deep sequencing. Although we identified hundreds of peptides reactive with each of five different mouse and human TCRs, the selected peptides possessed TCR recognition motifs that bore a close resemblance to their known antigens. This structural conservation of the TCR interaction surface allowed us to exploit deep-sequencing information to computationally identify activating microbial and self-ligands for human autoimmune TCRs. The mechanistic basis of TCR cross-reactivity described here enables effective surveillance of diverse self and foreign antigens without necessitating degenerate recognition of nonhomologous peptides.
- Departments of Molecular and Cellular Physiology and Structural Biology, Stanford University School of Medicine, Stanford, CA 94305, USA; Program in Immunology, Stanford University School of Medicine, Stanford, CA 94305, USA.
Organizational Affiliation: