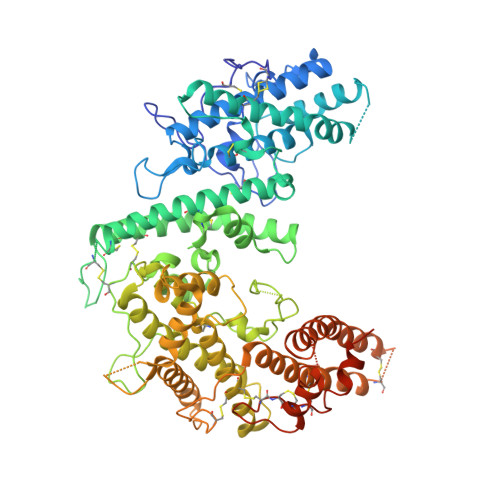
Structure of the DBL3X-DBL4 epsilon region of the VAR2CSA placental malaria vaccine candidate: insight into DBL domain interactions.
Gangnard, S., Lewit-Bentley, A., Dechavanne, S., Srivastava, A., Amirat, F., Bentley, G.A., Gamain, B.(2015) Sci Rep 5: 14868-14868
- PubMed: 26450557
- DOI: https://doi.org/10.1038/srep14868
- Primary Citation of Related Structures:
4P1T - PubMed Abstract:
The human malaria parasite, Plasmodium falciparum, is able to evade spleen-mediated clearing from blood stream by sequestering in peripheral organs. This is due to the adhesive properties conferred by the P. falciparum Erythrocyte Membrane Protein 1 (PfEMP1) family exported by the parasite to the surface of infected erythrocytes. Expression of the VAR2CSA variant of PfEMP1 leads to pregnancy-associated malaria, which occurs when infected erythrocytes massively sequester in the placenta by binding to low-sulfated Chondroitin Sulfate A (CSA) present in the intervillous spaces. VAR2CSA is a 350 kDa protein that carries six Duffy-Binding Like (DBL) domains, one Cysteine-rich Inter-Domain Regions (CIDR) and several inter-domain regions. In the present paper, we report for the first time the crystal structure at 2.9 Å of a VAR2CSA double domain, DBL3X-DBL4ε, from the FCR3 strain. DBL3X and DBL4ε share a large contact interface formed by residues that are invariant or highly conserved in VAR2CSA variants, which suggests that these two central DBL domains (DBL3X-DBL4ε) contribute significantly to the structuring of the functional VAR2CSA extracellular region. We have also examined the antigenicity of peptides corresponding to exposed loop regions of the DBL4ε structure.
- Inserm UMR_1134, Paris, France.
Organizational Affiliation: