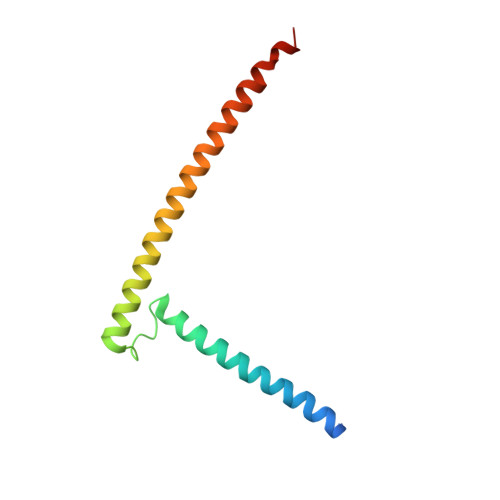Structural Basis for the Oligomerization of the MADS Domain Transcription Factor SEPALLATA3 in Arabidopsis.
Puranik, S., Acajjaoui, S., Conn, S., Costa, L., Conn, V., Vial, A., Marcellin, R., Melzer, R., Brown, E., Hart, D., Theien, G., Silva, C.S., Parcy, F., Dumas, R., Nanao, M., Zubieta, C.(2014) Plant Cell 26: 3603-3615
- PubMed: 25228343
- DOI: https://doi.org/10.1105/tpc.114.127910
- Primary Citation of Related Structures:
4OX0 - PubMed Abstract:
In plants, MADS domain transcription factors act as central regulators of diverse developmental pathways. In Arabidopsis thaliana, one of the most central members of this family is SEPALLATA3 (SEP3), which is involved in many aspects of plant reproduction, including floral meristem and floral organ development. SEP3 has been shown to form homo and heterooligomeric complexes with other MADS domain transcription factors through its intervening (I) and keratin-like (K) domains. SEP3 function depends on its ability to form specific protein-protein complexes; however, the atomic level determinants of oligomerization are poorly understood. Here, we report the 2.5-Å crystal structure of a small portion of the intervening and the complete keratin-like domain of SEP3. The domains form two amphipathic alpha helices separated by a rigid kink, which prevents intramolecular association and presents separate dimerization and tetramerization interfaces comprising predominantly hydrophobic patches. Mutations to the tetramerization interface demonstrate the importance of highly conserved hydrophobic residues for tetramer stability. Atomic force microscopy was used to show SEP3-DNA interactions and the role of oligomerization in DNA binding and conformation. Based on these data, the oligomerization patterns of the larger family of MADS domain transcription factors can be predicted and manipulated based on the primary sequence.
- European Synchrotron Radiation Facility, Structural Biology Group, 38042 Grenoble, France.
Organizational Affiliation: