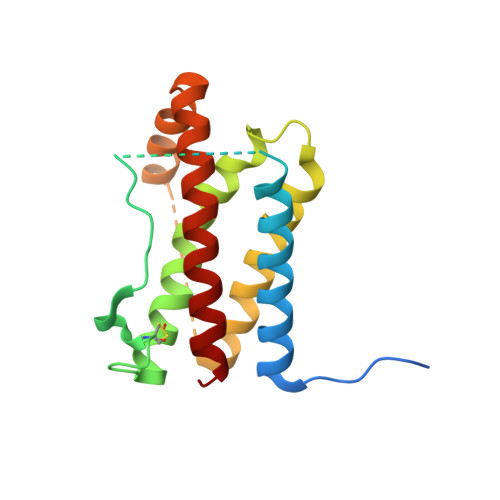
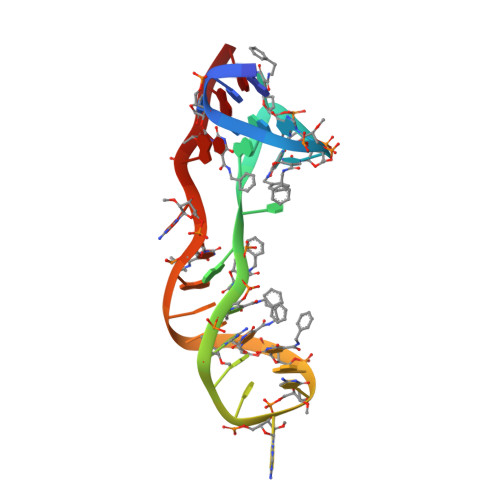
Crystal structure of interleukin-6 in complex with a modified nucleic Acid ligand.
Gelinas, A.D., Davies, D.R., Edwards, T.E., Rohloff, J.C., Carter, J.D., Zhang, C., Gupta, S., Ishikawa, Y., Hirota, M., Nakaishi, Y., Jarvis, T.C., Janjic, N.(2014) J Biological Chem 289: 8720-8734
- PubMed: 24415767
- DOI: https://doi.org/10.1074/jbc.M113.532697
- Primary Citation of Related Structures:
4NI7, 4NI9 - PubMed Abstract:
IL-6 is a secreted cytokine that functions through binding two cell surface receptors, IL-6Rα and gp130. Because of its involvement in the progression of several chronic inflammatory diseases, IL-6 is a target of pharmacologic interest. We have recently identified a novel class of ligands called SOMAmers (S low Off-rate Modified Aptamers) that bind IL-6 and inhibit its biologic activity. SOMAmers exploit the chemical diversity of protein-like side chains assembled on flexible nucleic acid scaffolds, resulting in an expanded repertoire of intra- and intermolecular interactions not achievable with conventional aptamers. Here, we report the co-crystal structure of a high affinity SOMAmer (Kd = 0.20 nm) modified at the 5-position of deoxyuridine in a complex with IL-6. The SOMAmer, comprised of a G-quartet domain and a stem-loop domain, engages IL-6 in a clamp-like manner over an extended surface exhibiting close shape complementarity with the protein. The interface is characterized by substantial hydrophobic interactions overlapping the binding surfaces of the IL-6Rα and gp130 receptors. The G-quartet domain retains considerable binding activity as a disconnected autonomous fragment (Kd = 270 nm). A single substitution from our diversely modified nucleotide library leads to a 37-fold enhancement in binding affinity of the G-quartet fragment (Kd = 7.4 nm). The ability to probe ligand surfaces in this manner is a powerful tool in the development of new therapeutic reagents with improved pharmacologic properties. The SOMAmer·IL-6 structure also expands our understanding of the diverse structural motifs achievable with modified nucleic acid libraries and elucidates the nature with which these unique ligands interact with their protein targets.
- From the SomaLogic, Inc., Boulder, Colorado 80301.
Organizational Affiliation: