Lipid ligands of human SPLUNC1
Ning, F., Wang, C., Niu, L., Chu, H.W., Zhang, G.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Maltose-binding periplasmic/Palate lung and nasal epithelium clone fusion protein | 584 | Escherichia coli, Homo sapiens This entity is chimeric | Mutation(s): 0 | 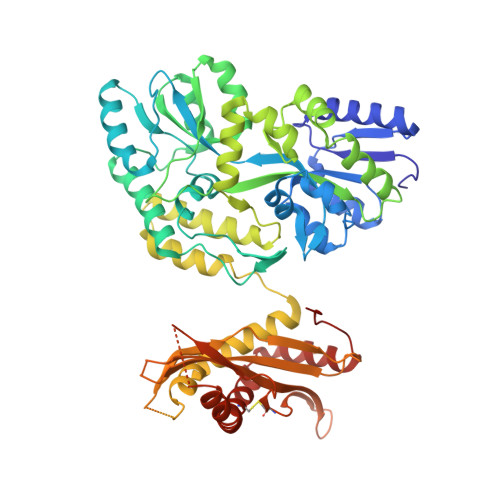 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0AEX9 (Escherichia coli (strain K12)) Explore P0AEX9 Go to UniProtKB: P0AEX9 | |||||
Find proteins for Q9NP55 (Homo sapiens) Explore Q9NP55 Go to UniProtKB: Q9NP55 | |||||
PHAROS: Q9NP55 GTEx: ENSG00000198183 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | Q9NP55P0AEX9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PEG Query on PEG | B [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| MG Query on MG | C [auth A] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 119.087 | α = 90 |
| b = 119.087 | β = 90 |
| c = 98.541 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| CNS | refinement |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| CNS | phasing |