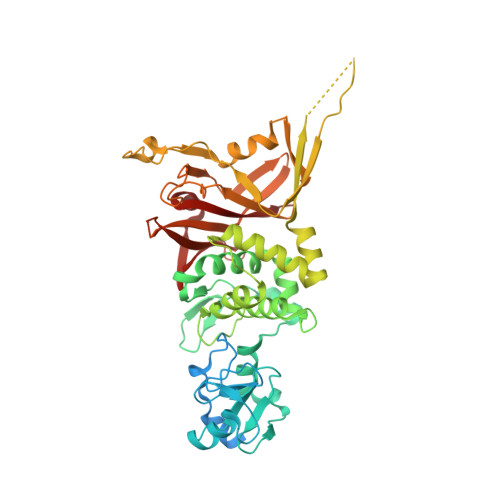
The crystal structure of S. cerevisiae Sad1, a catalytically inactive deubiquitinase that is broadly required for pre-mRNA splicing.
Hadjivassiliou, H., Rosenberg, O.S., Guthrie, C.(2014) RNA 20: 656-669
- PubMed: 24681967
- DOI: https://doi.org/10.1261/rna.042838.113
- Primary Citation of Related Structures:
4MSX - PubMed Abstract:
Sad1 is an essential splicing factor initially identified in a genetic screen in Saccharomyces cerevisiae for snRNP assembly defects. Based on sequence homology, Sad1, or USP39 in humans, is predicted to comprise two domains: a zinc finger ubiquitin binding domain (ZnF-UBP) and an inactive ubiquitin-specific protease (iUSP) domain, both of which are well conserved. The role of these domains in splicing and their interaction with ubiquitin are unknown. We first used splicing microarrays to analyze Sad1 function in vivo and found that Sad1 is critical for the splicing of nearly all yeast intron-containing genes. By using in vitro assays, we then showed that it is required for the assembly of the active spliceosome. To gain structural insights into Sad1 function, we determined the crystal structure of the full-length protein at 1.8 Å resolution. In the structure, the iUSP domain forms the characteristic ubiquitin binding pocket, though with an amino acid substitution in the active site that results in complete inactivation of the enzymatic activity of the domain. The ZnF-UBP domain of Sad1 shares high structural similarly to other ZnF-UBPs; however, Sad1's ZnF-UBP does not possess the canonical ubiquitin binding motif. Given the precedents for ZnF-UBP domains to function as activators for their neighboring USP domains, we propose that Sad1's ZnF-UBP acts in a ubiquitin-independent capacity to recruit and/or activate Sad1's iUSP domain to interact with the spliceosome.