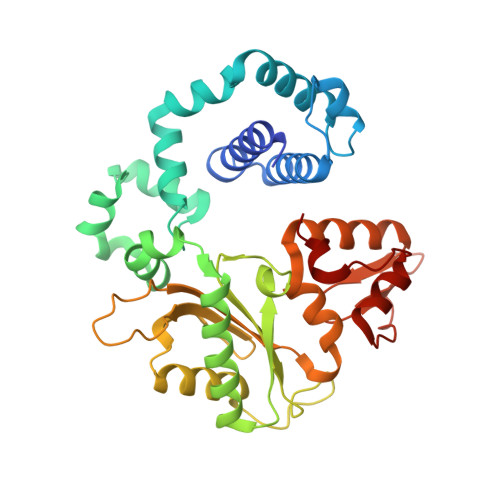
Observing a DNA polymerase choose right from wrong.
Freudenthal, B.D., Beard, W.A., Shock, D.D., Wilson, S.H.(2013) Cell 154: 157-168
- PubMed: 23827680
- DOI: https://doi.org/10.1016/j.cell.2013.05.048
- Primary Citation of Related Structures:
4KLD, 4KLE, 4KLF, 4KLG, 4KLH, 4KLI, 4KLJ, 4KLL, 4KLM, 4KLO, 4KLQ, 4KLS, 4KLT, 4KLU, 4LVS - PubMed Abstract:
DNA polymerase (pol) β is a model polymerase involved in gap-filling DNA synthesis utilizing two metals to facilitate nucleotidyl transfer. Previous structural studies have trapped catalytic intermediates by utilizing substrate analogs (dideoxy-terminated primer or nonhydrolysable incoming nucleotide). To identify additional intermediates during catalysis, we now employ natural substrates (correct and incorrect nucleotides) and follow product formation in real time with 15 different crystal structures. We are able to observe molecular adjustments at the active site that hasten correct nucleotide insertion and deter incorrect insertion not appreciated previously. A third metal binding site is transiently formed during correct, but not incorrect, nucleotide insertion. Additionally, long incubations indicate that pyrophosphate more easily dissociates after incorrect, compared to correct, nucleotide insertion. This appears to be coupled to subdomain repositioning that is required for catalytic activation/deactivation. The structures provide insights into a fundamental chemical reaction that impacts polymerase fidelity and genome stability.
- Laboratory of Structural Biology, National Institute of Environmental Health Sciences, National Institutes of Health, P.O. Box 12233, Research Triangle Park, NC 27709-2233, USA.
Organizational Affiliation: