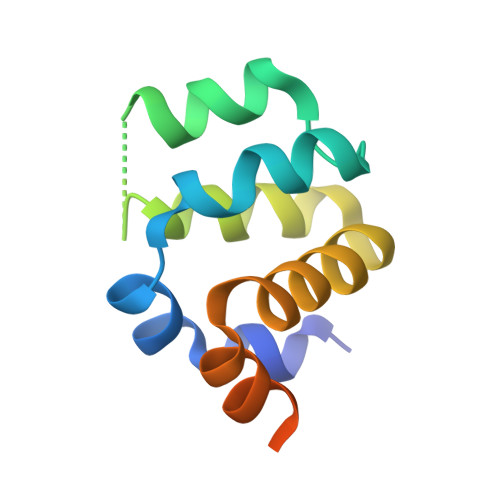
Novel Disulfide Bond-Mediated Dimerization of the CARD Domain Was Revealed by the Crystal Structure of CARMA1 CARD
Jang, T.H., Park, J.H., Park, H.H.(2013) PLoS One 8: e79778-e79778
- PubMed: 24224005
- DOI: https://doi.org/10.1371/journal.pone.0079778
- Primary Citation of Related Structures:
4JUP - PubMed Abstract:
CARMA1, BCL10 and MALT1 form a large molecular complex known as the CARMA1 signalosome during lymphocyte activation. Lymphocyte activation via the CARMA1 signalosome is critical to immune response and linked to many immune diseases. Despite the important role of the CARMA1 signalosome during lymphocyte activation and proliferation, limited structural information is available. Here, we report the dimeric structure of CARMA1 CARD at a resolution of 3.2 Å. Interestingly, although CARMA1 CARD has a canonical six helical-bundles structural fold similar to other CARDs, CARMA1 CARD shows the first homo-dimeric structure of CARD formed by a disulfide bond and reveals a possible biologically important homo-dimerization mechanism.
- School of Biotechnology and Graduate School of Biochemistry at Yeungnam University, Gyeongsan, South Korea.
Organizational Affiliation: