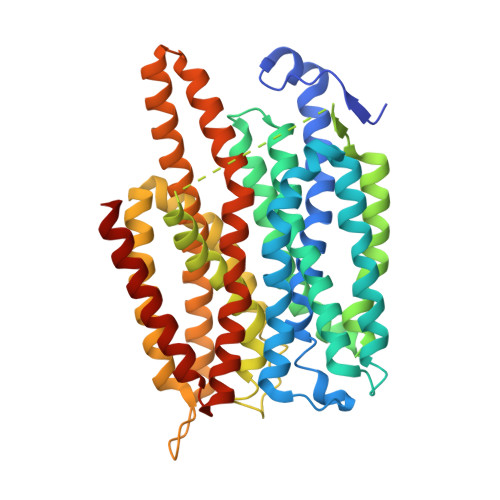
Crystal structure of a nitrate/nitrite exchanger.
Zheng, H., Wisedchaisri, G., Gonen, T.(2013) Nature 497: 647-651
- PubMed: 23665960
- DOI: https://doi.org/10.1038/nature12139
- Primary Citation of Related Structures:
4JR9, 4JRE - PubMed Abstract:
Mineral nitrogen in nature is often found in the form of nitrate (NO3(-)). Numerous microorganisms evolved to assimilate nitrate and use it as a major source of mineral nitrogen uptake. Nitrate, which is central in nitrogen metabolism, is first reduced to nitrite (NO2(-)) through a two-electron reduction reaction. The accumulation of cellular nitrite can be harmful because nitrite can be reduced to the cytotoxic nitric oxide. Instead, nitrite is rapidly removed from the cell by channels and transporters, or reduced to ammonium or dinitrogen through the action of assimilatory enzymes. Despite decades of effort no structure is currently available for any nitrate transport protein and the mechanism by which nitrate is transported remains largely unknown. Here we report the structure of a bacterial nitrate/nitrite transport protein, NarK, from Escherichia coli, with and without substrate. The structures reveal a positively charged substrate-translocation pathway lacking protonatable residues, suggesting that NarK functions as a nitrate/nitrite exchanger and that protons are unlikely to be co-transported. Conserved arginine residues comprise the substrate-binding pocket, which is formed by association of helices from the two halves of NarK. Key residues that are important for substrate recognition and transport are identified and related to extensive mutagenesis and functional studies. We propose that NarK exchanges nitrate for nitrite by a rocker switch mechanism facilitated by inter-domain hydrogen bond networks.
- Janelia Farm Research Campus, Howard Hughes Medical Institute, 19700 Helix Drive, Ashburn, Virginia 20147, USA.
Organizational Affiliation: