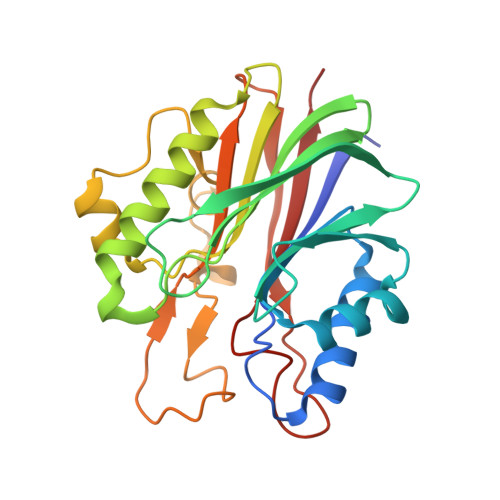
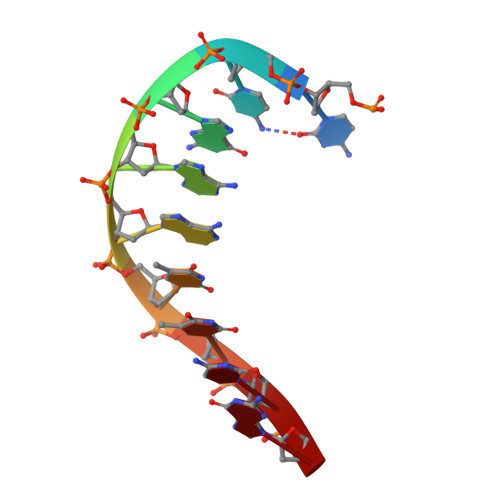
Mechanism of repair of 5'-topoisomerase II-DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2.
Schellenberg, M.J., Appel, C.D., Adhikari, S., Robertson, P.D., Ramsden, D.A., Williams, R.S.(2012) Nat Struct Mol Biol 19: 1363-1371
- PubMed: 23104055
- DOI: https://doi.org/10.1038/nsmb.2418
- Primary Citation of Related Structures:
4GYZ, 4GZ0, 4GZ1, 4GZ2 - PubMed Abstract:
The topoisomerase II (topo II) DNA incision-and-ligation cycle can be poisoned (for example following treatment with cancer chemotherapeutics) to generate cytotoxic DNA double-strand breaks (DSBs) with topo II covalently conjugated to DNA. Tyrosyl-DNA phosphodiesterase 2 (Tdp2) protects genomic integrity by reversing 5'-phosphotyrosyl-linked topo II-DNA adducts. Here, X-ray structures of mouse Tdp2-DNA complexes reveal that Tdp2 β-2-helix-β DNA damage-binding 'grasp', helical 'cap' and DNA lesion-binding elements fuse to form an elongated protein-DNA conjugate substrate-interaction groove. The Tdp2 DNA-binding surface is highly tailored for engagement of 5'-adducted single-stranded DNA ends and restricts nonspecific endonucleolytic or exonucleolytic processing. Structural, mutational and functional analyses support a single-metal ion catalytic mechanism for the exonuclease-endonuclease-phosphatase (EEP) nuclease superfamily and establish a molecular framework for targeted small-molecule blockade of Tdp2-mediated resistance to anticancer topoisomerase drugs.
- Laboratory of Structural Biology, National Institute of Environmental Health Sciences, US National Institutes of Health, Department of Health and Human Services, North Carolina, USA.
Organizational Affiliation: