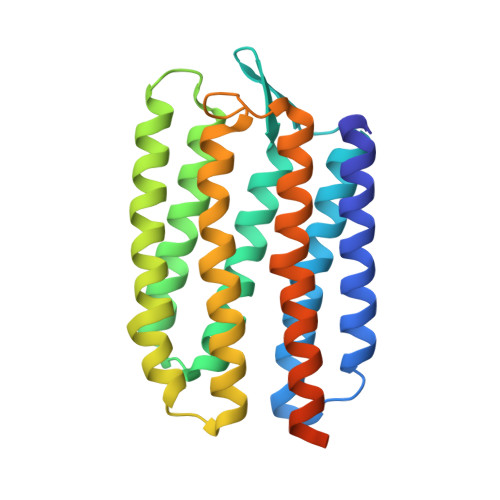
Ground state structure of D75N mutant of sensory rhodopsin II in complex with its cognate transducer.
Ishchenko, A., Round, E., Borshchevskiy, V., Grudinin, S., Gushchin, I., Klare, J.P., Balandin, T., Remeeva, A., Engelhard, M., Buldt, G., Gordeliy, V.(2013) J Photochem Photobiol B 123C: 55-58
- PubMed: 23619282
- DOI: https://doi.org/10.1016/j.jphotobiol.2013.03.008
- Primary Citation of Related Structures:
4GYC - PubMed Abstract:
The complex of sensory rhodopsin II (NpSRII) with its cognate transducer (NpHtrII) mediates negative phototaxis in halobacteria Natronomonas pharaonis. Upon light activation NpSRII triggers, by means of NpHtrII, a signal transduction chain homologous to the two component system in eubacterial chemotaxis. Here we report on the crystal structure of the ground state of the mutant NpSRII-D75N/NpHtrII complex in the space group I212121. Mutations of this aspartic acid in light-driven proton pumps dramatically modify or/and inhibit protein functions. However, in vivo studies show that the similar D75N mutation retains functionality of the NpSRII/NpHtrII complex. The structure provides the molecular basis for the explanation of the unexpected observation that the wild and the mutant complexes display identical physiological response on light excitation.
- Institute of Complex Systems (ICS), ICS-5: Molecular Biophysics, Research Centre Juelich, 52425 Juelich, Germany.
Organizational Affiliation: