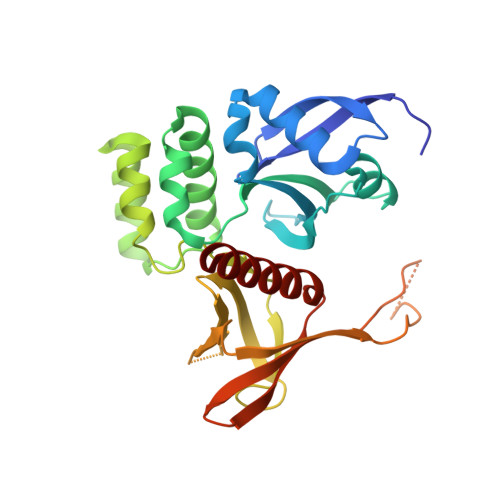
Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins.
Ghai, R., Bugarcic, A., Liu, H., Norwood, S.J., Skeldal, S., Coulson, E.J., Li, S.S., Teasdale, R.D., Collins, B.M.(2013) Proc Natl Acad Sci U S A 110: E643-E652
- PubMed: 23382219
- DOI: https://doi.org/10.1073/pnas.1216229110
- Primary Citation of Related Structures:
4GXB - PubMed Abstract:
Transit of proteins through the endosomal organelle following endocytosis is critical for regulating the homeostasis of cell-surface proteins and controlling signal transduction pathways. However, the mechanisms that control these membrane-transport processes are poorly understood. The Phox-homology (PX) domain-containing proteins sorting nexin (SNX) 17, SNX27, and SNX31 have emerged recently as key regulators of endosomal recycling and bind conserved Asn-Pro-Xaa-Tyr-sorting signals in transmembrane cargos via an atypical band, 4.1/ezrin/radixin/moesin (FERM) domain. Here we present the crystal structure of the SNX17 FERM domain bound to the sorting motif of the P-selectin adhesion protein, revealing both the architecture of the atypical FERM domain and the molecular basis for recognition of these essential sorting sequences. We further show that the PX-FERM proteins share a promiscuous ability to bind a wide array of putative cargo molecules, including receptor tyrosine kinases, and propose a model for their coordinated molecular interactions with membrane, cargo, and regulatory proteins.
- Institute for Molecular Bioscience, The University of Queensland, St. Lucia, QLD 4072, Australia.
Organizational Affiliation: