Crystal Structure of the first catalytic domain of protein disulfide isomerase P5
Vinaik, R., Kozlov, G., Gehring, K.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein disulfide-isomerase A6 | 123 | Homo sapiens | Mutation(s): 0 Gene Names: PDIA6, ERP5, P5, TXNDC7 EC: 5.3.4.1 | 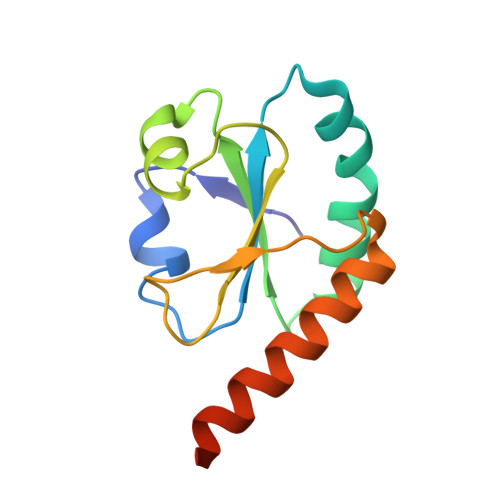 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q15084 (Homo sapiens) Explore Q15084 Go to UniProtKB: Q15084 | |||||
PHAROS: Q15084 GTEx: ENSG00000143870 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q15084 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 129.852 | α = 90 |
| b = 129.852 | β = 90 |
| c = 45.226 | γ = 120 |
| Software Name | Purpose |
|---|---|
| ADSC | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |