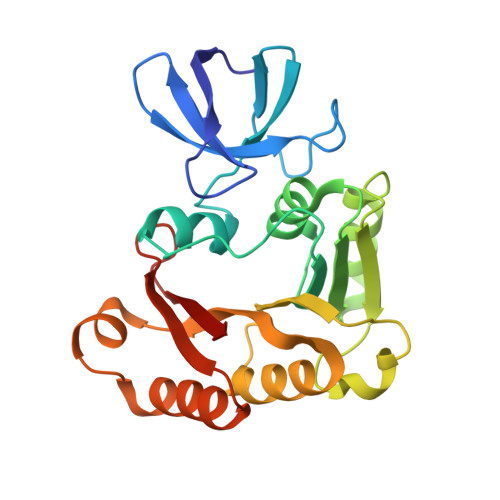
Structure of Aeropyrum pernix fibrillarin in complex with natively bound S-adenosyl-L-methionine at 1.7 A resolution.
de Silva, U., Zhou, Z., Brown, B.A.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 854-859
- PubMed: 22869109
- DOI: https://doi.org/10.1107/S1744309112026528
- Primary Citation of Related Structures:
4DF3 - PubMed Abstract:
Fibrillarin is the key methyltransferase associated with the C/D class of small nuclear ribonucleoproteins (snRNPs) and participates in the preliminary step of pre-ribosomal rRNA processing. This molecule is found in the fibrillar regions of the eukaryotic nucleolus and is involved in methylation of the 2'-O atom of ribose in rRNA. Human fibrillarin contains an N-terminal GAR domain, a central RNA-binding domain comprising an RNP-2-like superfamily consensus sequence and a catalytic C-terminal helical domain. Here, Aeropyrum pernix fibrillarin is described, which is homologous to the C-terminal domain of human fibrillarin. The protein was crystallized with an S-adenosyl-L-methionine (SAM) ligand bound in the active site. The molecular structure of this complex was solved using X-ray crystallography at a resolution of 1.7 Å using molecular replacement with fibrillarin structural homologs. The structure shows the atomic details of SAM and its active-site interactions; there are a number of conserved residues that interact directly with the cofactor. Notably, the adenine ring of SAM is stabilized by π-π interactions with the conserved residue Phe110 and by electrostatic interactions with the Asp134, Ala135 and Gln157 residues. The π-π interaction appears to play a critical role in stabilizing the association of SAM with fibrillarin. Furthermore, comparison of A. pernix fibrillarin with homologous structures revealed different orientations of Phe110 and changes in α-helix 6 of fibrillarin and suggests key differences in its interactions with the adenine ring of SAM in the active site and with the C/D RNA. These differences may play a key role in orienting the SAM ligand for catalysis as well as in the assembly of other ribonucleoproteins and in the interactions with C/D RNA.
- Laboratory of Molecular Biology, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 9000 Rockville Pike, Bethesda, MD 20892, USA.
Organizational Affiliation: