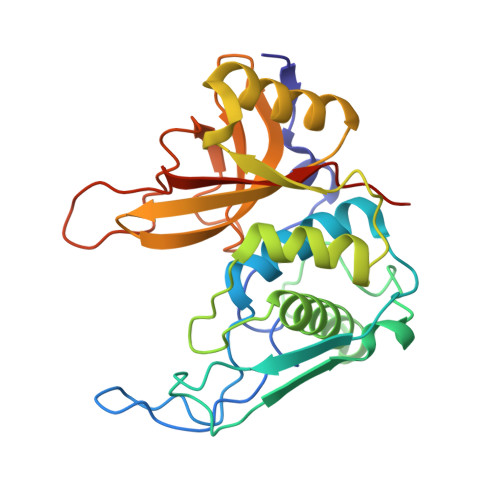
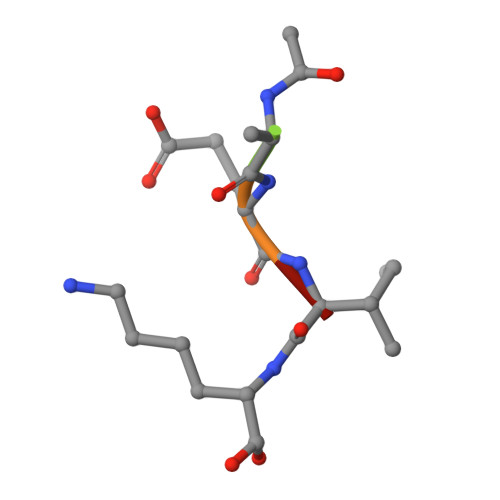
Ultrahigh and High Resolution Structures and Mutational Analysis of Monomeric Streptococcus pyogenes SpeB Reveal a Functional Role for the Glycine-rich C-terminal Loop.
Gonzalez-Paez, G.E., Wolan, D.W.(2012) J Biological Chem 287: 24412-24426
- PubMed: 22645124
- DOI: https://doi.org/10.1074/jbc.M112.361576
- Primary Citation of Related Structures:
4D8B, 4D8E, 4D8I - PubMed Abstract:
Cysteine protease SpeB is secreted from Streptococcus pyogenes and has been studied as a potential virulence factor since its identification almost 70 years ago. Here, we report the crystal structures of apo mature SpeB to 1.06 Å resolution as well as complexes with the general cysteine protease inhibitor trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane and a novel substrate mimetic peptide inhibitor. These structures uncover conformational changes associated with maturation of SpeB from the inactive zymogen to its active form and identify the residues required for substrate binding. With the use of a newly developed fluorogenic tripeptide substrate to measure SpeB activity, we determined IC(50) values for trans-epoxysuccinyl-l-leucylamido(4-guanidino)butane and our new peptide inhibitor and the effects of mutations within the C-terminal active site loop. The structures and mutational analysis suggest that the conformational movements of the glycine-rich C-terminal loop are important for the recognition and recruitment of biological substrates and release of hydrolyzed products.
- Department of Molecular and Experimental, The Scripps Research Institute, La Jolla, California 92037, USA.
Organizational Affiliation: