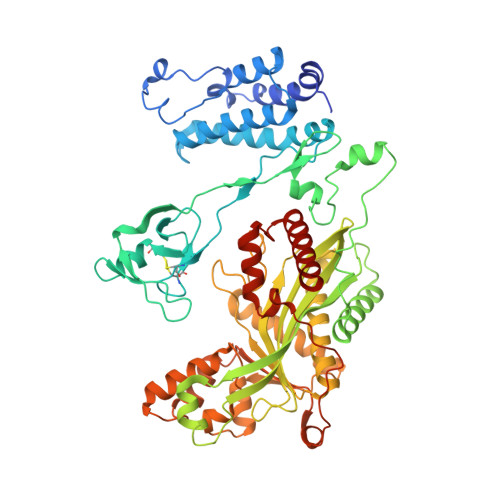
Structure of the P300 Catalytic Core and Implications for Chromatin Targeting and Hat Regulation
Delvecchio, M., Gaucher, J., Aguilar-Gurrieri, C., Ortega, E., Panne, D.(2013) Nat Struct Mol Biol 20: 1040
- PubMed: 23934153
- DOI: https://doi.org/10.1038/nsmb.2642
- Primary Citation of Related Structures:
4BHW - PubMed Abstract:
CBP and p300 are histone acetyltransferases (HATs) that associate with and acetylate transcriptional regulators and chromatin. Mutations in their catalytic 'cores' are linked to genetic disorders, including cancer. Here we present the 2.8-Å crystal structure of the catalytic core of human p300 containing its bromodomain, CH2 region and HAT domain. The structure reveals that the CH2 region contains a discontinuous PHD domain interrupted by a RING domain. The bromodomain, PHD, RING and HAT domains adopt an assembled configuration with the RING domain positioned over the HAT substrate-binding pocket. Disease mutations that disrupt RING attachment led to upregulation of HAT activity, thus revealing an inhibitory role for this domain. The structure provides a starting point for understanding how chromatin-substrate targeting and HAT regulation are coupled and why mutations in the p300 core lead to dysregulation.
- 1] European Molecular Biology Laboratory, Grenoble, France. [2] Unit for Virus Host-Cell Interactions, University of Grenoble Alpes, European Molecular Biology Laboratory-Centre National de la Recherche Scientifique, Grenoble, France. [3].
Organizational Affiliation: